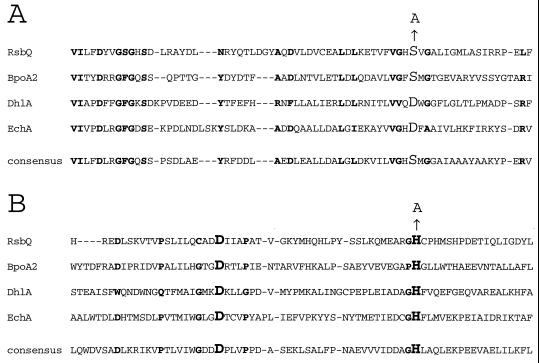
FIG. 4.
Partial alignment of RsbQ with well-characterized members of the α/β hydrolase superfamily. The crystal structures have been solved and the residues comprising the catalytic triads have been suggested or established for BpoA2, a bromoperoxidase from Streptomyces aureofaciens (16); DhlA, a haloalkane halidohydrolase from Xanthobacter autotrophicus (14, 28, 29, 39, 40); and EchA, an epoxide hydrolase from Agrobacterium radiobacter (24, 31). RsbQ and these other family members are aligned by the Reverse Position Blast algorithm (4) against the consensus for the α/β superfamily found in the Conserved Domain Database, maintained by the National Library of Medicine (www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). RsbQ was 23.5% identical to the α/β hydrolase fold consensus over a 226-residue overlap (E = 3 × 10−15). The two regions surrounding the proposed catalytic triad residues are shown here. Residues in boldface indicate the positions conserved in at least three of the four proteins. (A) Residues surrounding the proposed nucleophile S96 of RsbQ, shown here in larger font together with the S→A substitution. The catalytic nucleophiles in the other proteins and in the consensus are also identified by the larger font. (B) Residues surrounding the proposed acidic residue D219 and the proposed histidine residue H247 of RsbQ, shown here in larger font together with the H→A substitution at H247. The catalytic acidic and histidine residues in the other proteins and in the consensus are also identified by the larger font.