FIGURE 1.
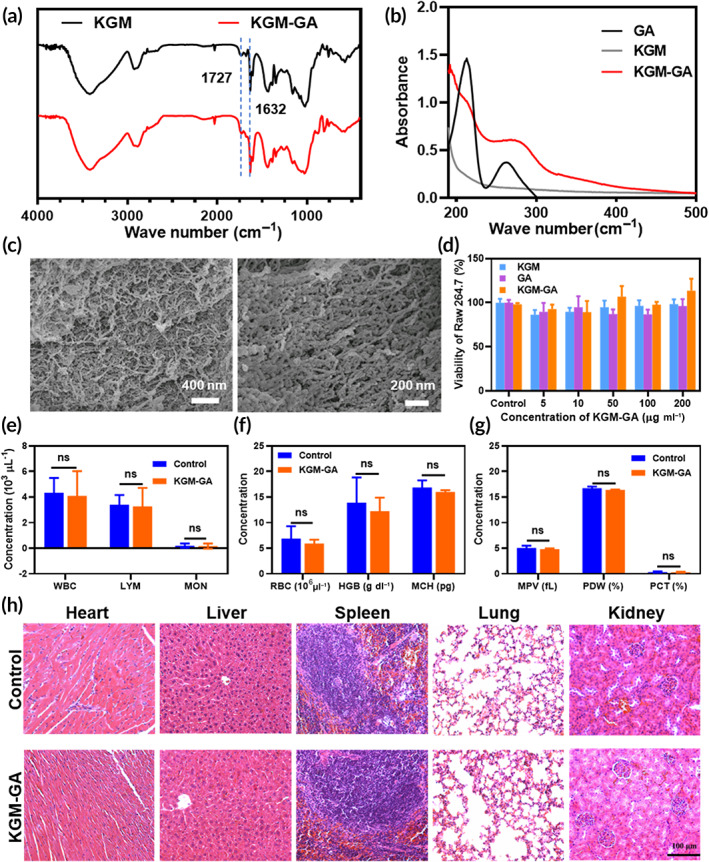
Structural characterization and biocompatibility assessments. (a) Fourier transform infrared (FTIR) spectrum of konjac glucomannan (KGM) and KGM‐GA. (b) UV–vis spectra of gallic acid (GA), KGM, and KGM‐GA. (c) Scanning electron microscopy (SEM) image of KGM‐GA. (d) Viability of Raw 264.7 cells treated with KGM, GA, and KGM‐GA, respectively, for 24 h at different concentrations. (e–g) Blood parameters in normal mice (control group), and mice treated with KGM‐GA after 14 days. (h) Evaluation of in vivo toxicity of KGM‐GA to major organs (heart, liver, spleen, lung, and kidney) at 14 days after treatment compared with normal mice (control group). The data were conducted by two‐way ANOVA with Sidak's post hoc test. The data are represented by means ± SD (n = 3, ns p > 0.05).
