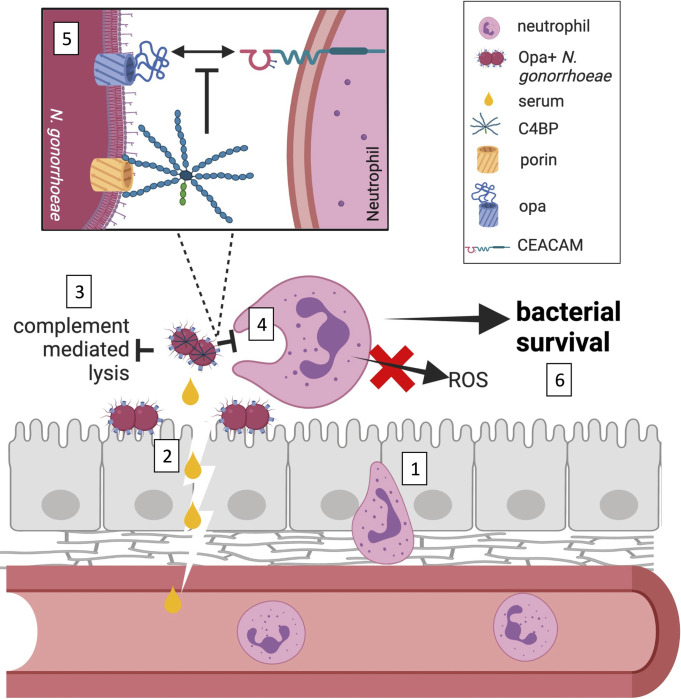
Fig 7. C4BP in gonococcal pathogenesis.
Neutrophils are recruited to mucosal sites of Neisseria gonorrhoeae infection by extravasating from the blood stream and crossing the epithelia paracellularly (1). Inflammatory conditions of the infection as well as serum transudate bring N. gonorrhoeae into contact with C4b-binding protein (C4BP) (2). The ability of N. gonorrhoeae to bind to C4BP to inhibit complement-mediated lysis has been extensively documented (3). This work shows that C4BP that is bound to the surface of N. gonorrhoeae decreases phagocytic uptake by neutrophils and suppresses neutrophil reactive oxygen species (ROS) production, in a complement-independent manner (4). C4BP binding decreases the interaction of CEACAM-binding, opacity protein (Opa)-expressing N. gonorrhoeae with neutrophils, but not bacteria that are opsonized with IgG or that express Opa proteins that engage other ligands (5). As a consequence, C4BP enhances survival of N. gonorrhoeae from neutrophils, a novel function for this canonical complement inhibitor (6). Figure generated using Biorender (https://biorender.com/).