Abstract
Although antiretroviral therapy (ART) has increased the quality of life and lifespan in people living with HIV (PWH), millions continue to suffer from the neurobehavioral effects of the virus. Additionally, the abuse of illicit drugs (methamphetamine in particular) is significantly higher in PWH compared to the general population, which may further impact their neurological functions. The HIV regulatory protein, Tat, has been implicated in the neurobehavioral impacts of HIV and is purported to inhibit dopamine transporter (DAT) function in a way similar to methamphetamine. Thus, we hypothesized that a combination of Tat expression and methamphetamine would exert synergistic deleterious effects on behavior and DAT expression. We examined the impact of chronic methamphetamine exposure on exploration in transgenic mice expressing human Tat (iTat) vs. their wildtype littermates using the behavioral pattern monitor (BPM).
During baseline, mice exhibited sex-dependent differences in BPM behavior, which persisted through methamphetamine exposure, and Tat activation with doxycycline. We observed a main effect of methamphetamine, wherein exposure, irrespective of genotype, increased locomotor activity and decreased specific exploration. After doxycycline treatment, mice continued to exhibit drug-dependent alterations in locomotion, with no effect of Tat, or methamphetamine interactions. DAT levels were higher in wildtype, saline-exposed males compared to all other groups.
These data support stimulant-induced changes of locomotor activity and exploration, and suggest that viral Tat and methamphetamine do not synergistically interact to alter these behaviors in mice. These findings are important for future studies attempting to disentangle the effect of substances that impact DAT on HAND-relevant behaviors using such transgenic animals.
Keywords: iTAT HIV model, Behavioral pattern monitor, Mouse, Movement, Exploration, Dopamine
1. Introduction
The Joint United Nations Programme on HIV/AIDS estimates approximately 76 million people have been infected with HIV since the beginning of the epidemic (UNAIDS, 2020). Though successful antiretroviral therapy (ART) has reduced viral progression and mortality in people living with HIV (PWH), HIV-associated neurocognitive disorder (HAND) persists (Wang et al., 2020). Further, PWH use illicit drugs (e.g. methamphetamine) at higher rates than the general population (Clark et al., 2012; Mitchell et al., 2006), which are also known to impair cognitive and motor performance. Thus, it has been proposed that drug use may interact with the HIV virus to produce synergistic effects on physiology and behavior (see Mediouni et al., 2015; Reiner et al., 2009 for reviews). Indeed, cocaine use exacerbated response inhibition deficits (Wakim et al., 2022), and previous methamphetamine use disorder was associated with worsened sensorimotor and memory deficits (Walter et al., 2021a; Walter et al., 2021b) in PWH.
Transgenic rodents expressing HIV-relevant genes have physiological and behavioral phenotypes akin to PWH, and are invaluable tools for determining the behavioral impact of substance use and HIV, and contributing mechanisms. Specific proteins relevant to HIV have been associated with neurobehavioral changes, particularly the envelope protein, gp120, and the regulatory protein, Tat (see Gaskill et al., 2017; Thaney et al., 2018 for reviews). Transgenic rodent models expressing these viral proteins have revealed that both gp120 and Tat interact with methamphetamine exposure to effect cognitive and motor deficits in mice (Baek et al., 2020; Henry et al., 2013; Kesby et al., 2016; Kesby et al., 2015; Kesby et al., 2017; Liu et al., 2014; Walter et al., 2021b). The exact mechanism by which HIV and methamphetamine interact to alter behavior however, remains unknown.
HAND deficits span motor and cognitive domains, suggesting dysfunctional dopamine (DA) signaling as a contributing factor. Indeed, cerebrospinal fluid dopamine levels are lower in PWH (Berger et al., 1994) and dopamine transporter (DAT) availability is largely decreased in deep cortical tissues integral for cognitive and motor function (i.e. basal ganglia) in both HAND (Chang et al., 2008), and HIV-associated dementia diagnosed (Wang et al., 2004) PWH. Lower DAT levels in the basal ganglia were also associated with enhanced memory and psychomotor impairments in HAND diagnosed PWH (Chang et al., 2008). Like methamphetamine, the Tat protein alters DA via the inhibition of DAT, which may contribute to their interactive effects on behavior. In fact, Appadoo et al. (2017) demonstrated the combination of Tat and methamphetamine exposure decreased DAT function further than either manipulation alone, though no behavior was assessed in this study. Thus, DAT inhibition may be a shared mechanism by which methamphetamine exposure and the Tat protein alter synergistically alter behavior.
Locomotor activity and exploration are largely altered by DAT inhibition (e.g., methamphetamine exposure), and HIV protein exposure in mice, as measured in the behavioral pattern monitor (BPM) task (Henry et al., 2013; Kwiatkowski et al., 2019; van Enkhuizen et al., 2014; Young et al., 2010b). Given the importance of activity to measuring cognitive performance, here, we used the inducible-Tat (iTat) transgenic mouse model (Kim et al., 2003) to determine the individual and combined effects of doxycycline-induced Tat expression and chronic methamphetamine exposure on locomotor and exploratory activity in the BPM. The iTat model was chosen as it produces Tat expression in the brain similar to levels observed in the brains of PWH, and recreates physiological and behavioral phenotypes relevant to HIV viral progression, including, impaired astrocyte proliferation and neurogenesis, as well as cognitive and motor impairments (Langford et al., 2018). Additionally, unlike congenital models (e.g., gp120 mice), the protein expression in the iTat model can be induced at time points more consistent with human HIV onset, which can clarify the impact of Tat at specifically-timed experimental manipulations (i.e. following drug exposure). We further assessed the long-term effects of methamphetamine exposure and Tat expression on DAT expression via immunostaining. We hypothesized that, while both methamphetamine and Tat alone would alter BPM measures, their combined effects would synergistically alter activity and exploration. Additionally, we hypothesized that iTat mice exposed to methamphetamine would exhibit reduced numbers of DAT-positive cells in brain regions associated with locomotion and exploratory behavior.
2. Methods
2.1. Animals
Male and female adult mice (Baseline Experiment: 30 WT females/31 WT males, 29 iTat females/29 iTat males; Post- methamphetamine Experiment: 30 WT females/30 WT males, 29 iTat females/29 iTat males; Post-induced TAT expression Experiment: 30 WT females/27 WT males, 28 iTat females/24 iTat males; immunohistochemistry: 28 WT females/29 WT males, 22 iTat females/28 iTat males) on a C57Bl/6 J background were housed one-to-four mice per cage and maintained in a temperature-controlled (21 ± 1 °C) vivarium on a 12 h/12 h reversed light-dark cycle (1900 Lights On, 0700 Lights Off). Mice were 14 weeks old at the beginning of testing. Varied housing levels were a result of severe cage-mate aggression (single housing was in the vast minority). Mice lacking Tat (WT) contained GFAP promoter-controlled Tet-binding protein (TGFAP+) while mice expressing Tat (iTat) contained TGFAP+ promotor and the TRE promotor-Tat protein transgene (TAT86+). All mice were supplied by Dr. Marcus Kaul (UC Riverside) and kept in quarantine for 8 weeks prior to the beginning of training. All mice were given ad libitum access to water and were food restricted to approximately 85 % of their free-feeding body weight at least one week before the start training. Testing occurred in the dark phase (1300 to 1800). All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care.
2.2. Drug regimens
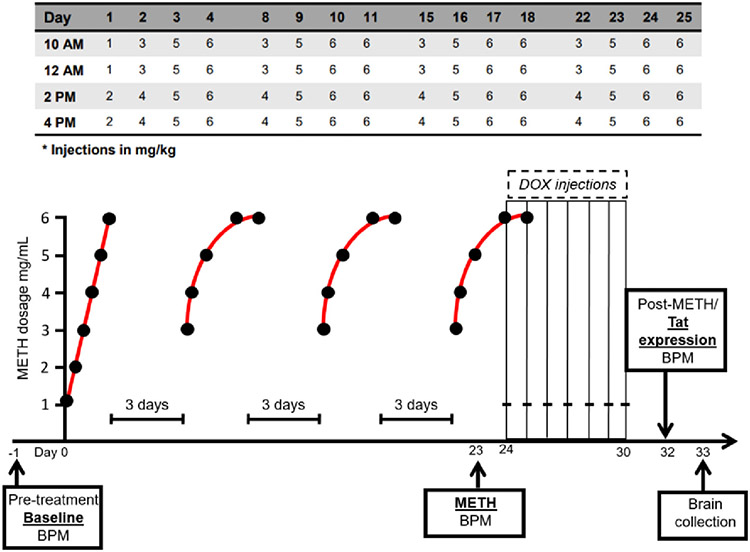
Methamphetamine was administered following previously described dosing regimens (Kesby et al., 2018a; Kesby et al., 2019; Kesby et al., 2018b), as this method fits models of total use per day per month in humans. Briefly, mice were administered subcutaneous injections of either 0.9 % saline or methamphetamine hydrochloride (5 ml/kg; Sigma, St. Louis, MO, USA) for 25 days (4-day block of injections separated by 3 days of washout; 4 injections/day at 1200, 1400, 1600, and 1800). Drug concentrations were gradually increased over four day injection blocks to produce binge-like exposure (Fig. 1).
Fig. 1.
Experimental timeline. Mice were assessed on the behavior pattern monitor (BPM) prior to any exposure to either methamphetamine (METH) or doxycycline (DOX)-induced Tat expression to establish a behavioral baseline. When the METH exposure regimen began, mice were treated with a low dose (1 mg/ml) and slowly increased to a higher dose (6 mg/ml). After the first four-day block of injections, injections began with a mid-range dose (3 mg/mL) and ended with the highest dose (6 mg/ml). Mice were treated four days in a row at four different time points (1000, 1200, 1400, 1600 h) separated by a three-day period without injections. The four-day block of injection occurred four separate times and the entire regimen lasted 25 days. Mice were further assessed on the BPM near the end of the METH regimen (day 23) and on Day 32 (2 days after the end of DOX injections and 7 days following METH exposure).
Beginning on Day 24 of the methamphetamine regimen, mice were given intraperitoneal injections of 100 mg/kg doxycycline (DOX; doxycycline hyclate; Sigma, St. Louis, MO, USA) daily for 7 days (days 24–30), paired with the first daily injection of the methamphetamine regimen (i.e., at 12:00), consistent with previous studies (Walter et al., 2021b) (Fig. 1). DOX was used to induce Tat expression in the iTat mice, while having no effect in WT mice, to assess the effects of Tat after heavy methamphetamine use. During the 25-day period, mice were weighed twice per week to determine the injection volume for the following days.
2.3. Behavioral pattern monitor
The mouse BPM, used to record animal movement and exploratory behavior, consisted of a Plexiglas chamber (area: 30.5 × 61 × 38 cm) illuminated by a single light source located above the arena (Kwiatkowski et al., 2019; Risbrough et al., 2006; van Enkhuizen et al., 2013). The chamber contained 3 floor holes and 8 wall holes and was equipped with 12 × 24 infrared photobeams 1 cm above the floor (2.5 cm apart) to detect holepoking behavior. A set of 16 infrared photobeams located 2.5 cm above the floor was used to detect rearing behavior. Mouse location was recorded every 0.1 s with its position defined across nine unequal regions (center, 4 corners, 4 walls). At the beginning of each session, mice were placed at the top left-hand corner of the chamber, facing the corner. Activity was measure using the 12 × 24 infrared photobeams 1 cm above the floor (2.5 cm apart). Behavioral measures have been defined previously (Cope et al., 2021) and were recorded during a 30 min session.
The primary outcomes measured included total behavioral activity (total number of distinct behaviors during testing; “counts”), transition behavior (crossing from one predefined area to another), travel distance (total distance traveled in cm), holepoking (total number of pokes across all 11 holes), rearing (number of movements in the y-axis), and spatial d (index of movement linearity with values closer to 1 reflecting straighter path movement and values closer to 2 indicating highly circumscribed movement; Paulus and Geyer, 1991). Testing occurred: 1) at baseline prior to drug administration 2) on day 23 at least 1 h following METH exposure and 3) on day 32 following TAT expression (Fig. 1).
2.4. Histology
Mouse brain hemispheres were collected one day following the last BPM test and fixed for 24 h in 4 % paraformaldehyde and embedded in paraffin. Briefly, as previously described (Kesby et al., 2018b), mouse hemibrain tissue sections (5 μm thickness) were deparaffinized using xylene followed by rehydration in serial ethanol and water solutions. Next, tissue sections were treated for 30 min with 3 % hydrogen peroxide/phosphate-buffered saline (PBS) and then incubated for 30 min with 2.5 % normal serum, corresponding to the host species for the secondary antibody. Tissue sections were then incubated with anti-DAT (Santa Cruz Biotechnology, Dallas, TX; Cat# sc- 32258; 1:100 in PBS) for 2 h at room temperature in a hydration box. Subsequently, tissue sections were washed with 0.1 % Tween 20/PBS, before 30 min incubation with horse anti-rabbit IgG peroxidase-polymer secondary antibody (ImmPRESS, Vector Laboratories, Burlingame, CA, USA). After the tissues were washed with 0.1 % Tween 20/PBS, the signals were developed with diaminobenzidine (ImmPACT DAB peroxidase substrate, Vector Laboratories) for 5 min. The immunostained sections were then dehydrated via serial ethanol and water solutions, dewaxed with xylene, and mounted using Cytoseal 60 (ThermoScientific). For the negative control, the primary antibody was omitted.
Subsequently, immunostained sections were scanned using a microscope slide scanner (Aperio ScanScope GL, Leica Biosystems, Buffalo Grove, IL, USA) equipped with a 20 × objective lens (yielding the resolution of 0.5 μm per pixel). Assessment of levels of DAT im- munoreactivity was performed using the Aperio ImageScope software. For each case a total of three sections (5 images per section) were analyzed to estimate the average optical density of immunolabelled cells per unit area (mm2). Corrected optical density was calculated by subtracting the background optical density of the negative control (obtained from tissue sections immunostained in the absence of primary antibody) from the optical density of the immunostained sections.
2.5. Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics 27 (Armonk, NY, USA). Behavioral and molecular data were analyzed using mixed analysis of variance (ANOVA), with drug and genotype as between-subjects factors. Results are expressed as mean ± standard error of the mean (SEM). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. BPM baseline
Mouse movement and exploration (primary measures: behavioral counts, transitions, total distance traveled, hole pokes, rearing, and spatial d) were measured in the BPM.
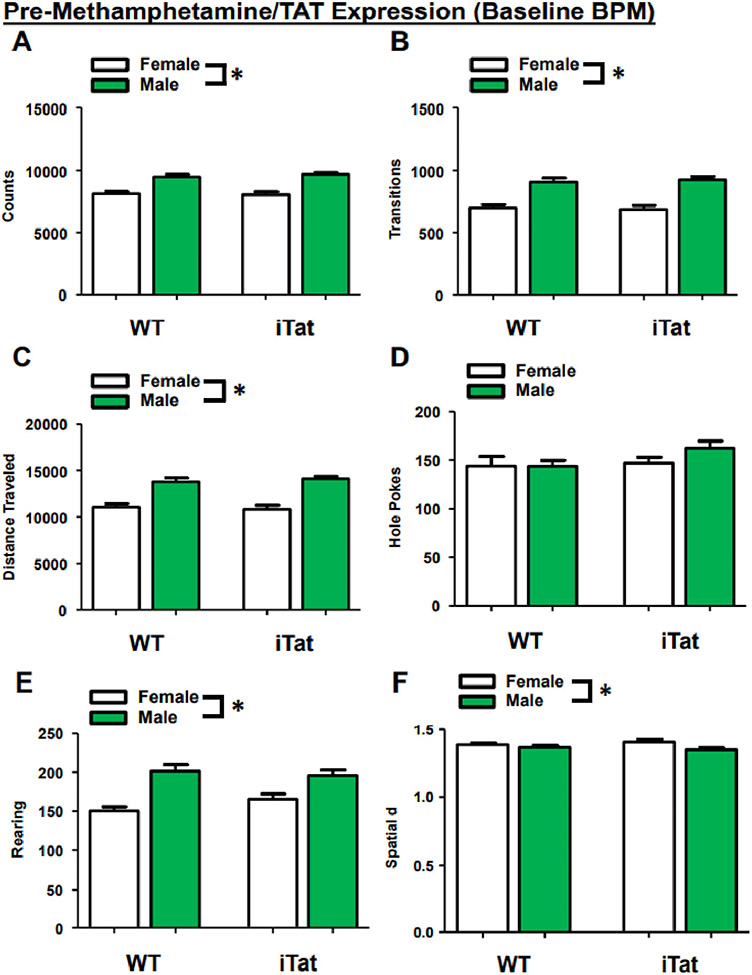
During Baseline, mice exhibited a main effect of sex on several measures including counts(F(1,111) = 65.874, p < 0.001; Fig. 2A), transitions (F(1,111) = 53.004, p < 0.001; Fig. 2B), distance traveled (F(1,111) = 61.516, p < 0.001; Fig. 2C), rearing (F(1,111) = 33.586, p < 0.001; Fig. 2E), and spatial d (F(1,111) = 7.292, p = 0.008; Fig. 2F), with female mice performing fewer of these activities and having a higher spatial d compared to males. There was no main effect of sex on holepoking (F(1,111) = 0.921, p = 0.339; Fig. 2D). There were no main effects of gene (counts: (F(1,111) = 0.186, p = 0.667; transitions: (F(1,111) = 0.075, p = 0.784; distance traveled: F(1,111) = 0.020, p = 0.888; holepoking: F(1,111) = 2.102, p = 0.150; rearing: F(1,111) = 0.417, p = 0.520; spatial d: F(1,111) = 0.001, p = 0.974) or drug (counts: F(1,111) = 0.001, p = 0.972; transitions: F(1,111) = 0.013, p = 0.908; distance traveled: F(1,111) = 0.001, p = 0.970; holepoking: F(1,111) = 0.000, p = 0.983; rearing: F(1,111) = 0.883, p = 0.350; spatial d: F(1,111) = 0.372, p = 0.543). The lack of main effects are important given that this testing period was before DOX-induced Tat expression or methamphetamine treatment, confirming consistency before treatment. There were also no interactions between any of the groups (ps > 0.1).
Fig. 2.
Sex determines baseline BPM activity, regardless of Tat promotor transgene expression. Compared to female mice, male mice exhibit higher total behavioral activity (A), transitions (B), and travel distance (C). Mice have similar holepoking behavior regardless of sex (D). While male mice exhibit higher rearing behavior (F), female mice have higher spatial d (F). Data presented as mean ± SEM. * = p < 0.05.
3.2. Effects of 23-day methamphetamine binge exposure on BPM behavior in iTat mice
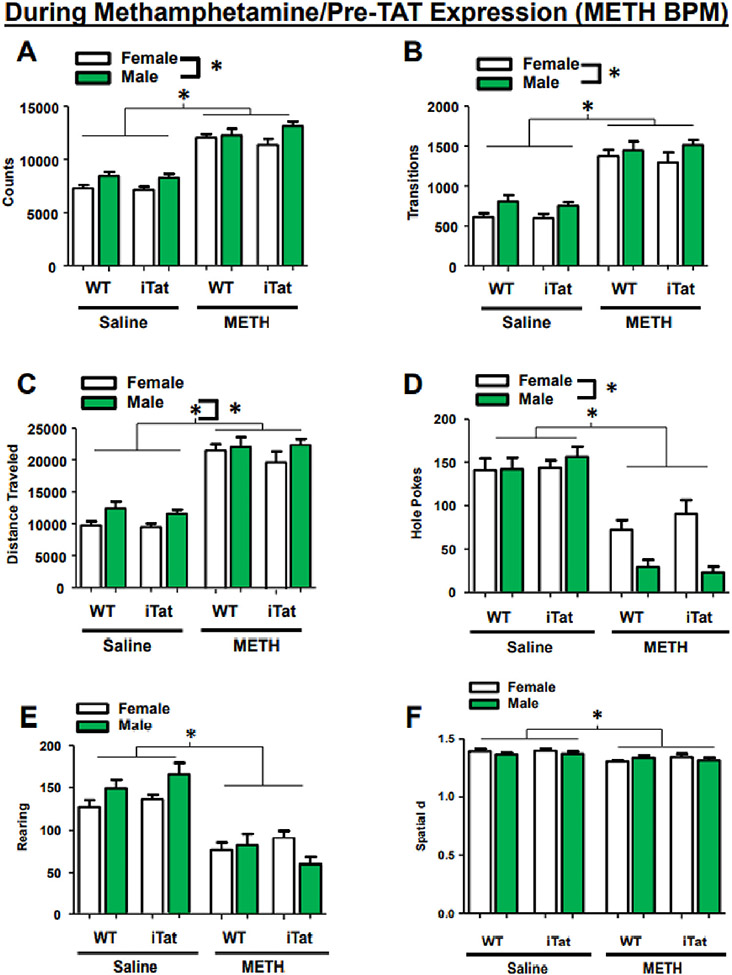
On day 23, at least 1 h following the first methamphetamine injection, mice were once again tested in the BPM. Across all behavioral measures, we observed a main effect of drug (F(1,110) = 225.895, p < 0.001) and sex (F(1,110) = 15.044, p < 0.001), with all males, and all mice previously exposed to METH, exhibiting more counts overall (Fig. 3A-F). Additionally, there was a sex × drug interaction in total counts (F(1,110) = 7.671, p = 0.007; Fig. 3A). Similarly, transitions and distanced traveled showed a main effect of drug (transitions: F(1,110) = 161.555, p < 0.001; Fig. 3B; distanced traveled: F(1,110) = 197.111, p < 0.001; Fig. 3C), with methamphetamine-exposed mice exhibiting increased activity. There was also a main effect of sex in transitions (F(1,110) = 8.820, p = 0.004) and distanced traveled (F(1,110) = 6.444, p = 0.013), with males exhibiting more of each behavior. For hole poking, female mice, overall, exhibited more activity (main effect of sex: F(1,110) = 9.065, p = 0.003), while, methamphetamine-exposed mice exhibited decreased holepoking (main effect of drug: F(1,110) = 128.837, p < 0.001; sex × drug interaction: F(1,110) = 128.837, p < 0.001; Fig. 3D). Finally, methamphetamine-exposed mice exhibited decreased rearing (F(1,110) = 89.818, p < 0.001; Fig. 3E) and spatial d F(1,110) = 15.453, p < 0.001; Fig. 3F) compared to control, with rearing showing an additional sex × drug interaction (F(1,110) = 7.671, p = 0.007).
Fig. 3.
Previous exposure to METH alters activity in the BPM. Mice previously exposed to METH exhibited higher total behavioral activity (A), transitions (B), and travel distance (C), with all males showing higher scores compared to females across all groups. METH exposure also reduced holepoking behavior (D), rearing (E), and spatial d (F). Holepoking behavior showed a sex × drug interaction, with male mice performing significantly lower than female mice (D). Data presented as mean ± SEM. * = p < 0.05.
3.3. Effects of TAT expression and post-methamphetamine binge on BPM activity in iTat mice
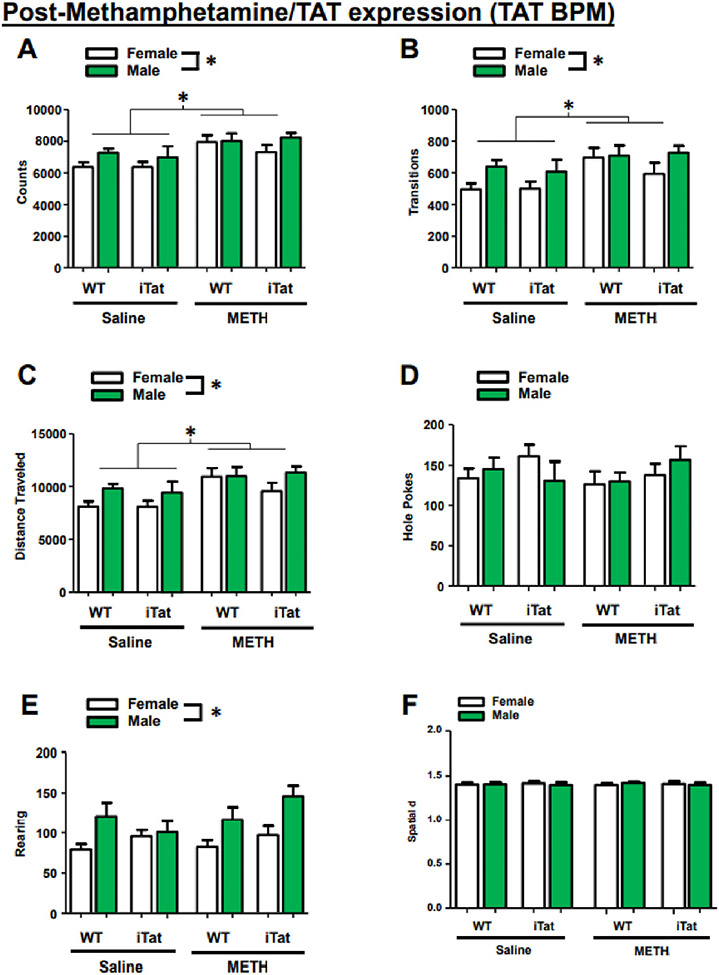
Seven days after the last methamphetamine exposure and 2 days after the last DOX administration (Day 32), mice were tested in the BPM. Overall, the primary measurements of the BPM were not affected by Tat expression.
During the final assessment, methamphetamine-exposed mice and all male mice exhibited higher total counts (drug: F(1,101) = 14.600, p < 0.001; sex: F(1,101) = 4.620, p = 0.034; Fig. 4A), higher transitions (drug: F(1,101) = 9.319, p = 0.003; sex: F(1,101) = 6.131, p = 0.015; Fig. 4B), and a longer distanced traveled (drug: F(1,101) = 13.072, p < 0.001; sex: F(1,101) = 5.994, p = 0.016; Fig. 4C), with no interactions between any groups. Lastly, while all male mice exhibited higher rearing (F(1,101) = 14.506, p < 0.001), there were no main effects or interactions in either holepoking or spatial d (Fig. 4D-F).
Fig. 4.
Movement in the BPM remains elevated long after METH exposure, but performance is unaltered by Tat expression. Five days after the end of the METH exposure regimen, METH-treated mice (and males across all groups) continue to display elevated total behavioral counts (A), transitions (B), and travel distance (C). METH treatment and Tat expression do not appear to affect holepokes (D), rearing (E), or spatial d (F). However, males across all groups exhibit higher rearing behavior than females (E). Data presented as mean ± SEM. * = p < 0.05.
3.4. DAT expression after TAT expression in methamphetamine-exposed mice
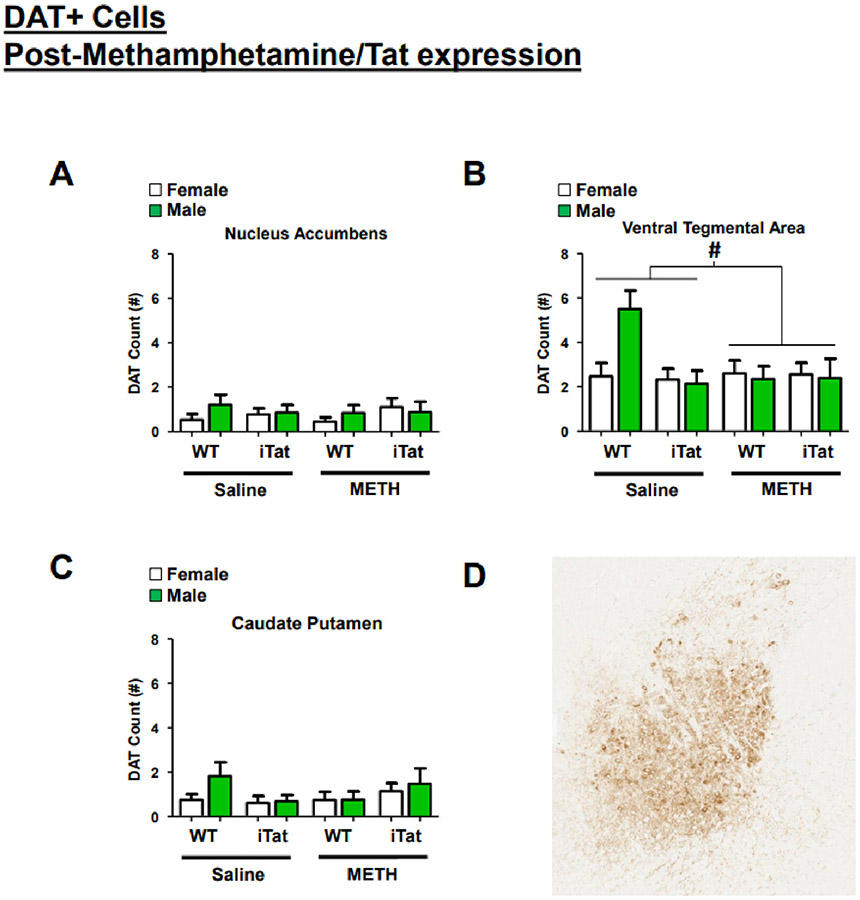
There were no main effects sex (F(1,99)=0.864, p = 0.355), gene (F(1,99)=0.011, p = 0.918) or drug (F(1,99)=0.039, p = 0.562) on DAT expression in the nucleus accumbens (Fig. 5A). While there were no significant effects in the ventral tegmental area, we observed that methamphetamine treatment tended to be associated with reduced DAT (F(1,99)=3.408, p = 0.068) driven primarily by higher levels in male WT saline-exposed mice (trend interactions: gene × drug: F(1,99)=3.467, p = 0.066; sex × gene: F(1,99)=3.029, p = 0.0858; sex × gene × drug: F(1,99)=3.075, p = 0.083; Fig. 5B). In the caudate putamen, we observed no main effect of sex (F(1,99)=1.357, p = 0.247), gene (F(1,99)=0.037, p = 0.847), or drug (F(1,99)=0.012, p = 0.912; Fig. 5C). We found, however, that gene and drug tended to interact (F(1,99)=3.541, p = 0.063) with both female WT saline- and female iTat METH-exposed mice exhibiting higher DAT.
Fig. 5.
DAT expression is slightly altered in the ventral tegmental area. After the third and final BPM assessment, mice were sacrificed and brain were taken for immunostaining to determine the effects of METH exposure and Tat expression on the dopamine transporter (DAT). No effects were seen on DAT expression in the nucleus accumbens (A). In the ventral tegmental area, saline-treated mice tended to have higher DAT expression, driven mainly by WT, male mice (B). Neither sex, METH exposure, nor Tat expression altered DAT expression in the caudate putamen (C). A representative image of immunohistochemistry for DAT (brown) on a paraffin embedded mouse brain, sagittal section, ventral tegmental area. Original magnification 5×; no counterstaining (D). Data presented as mean ± SEM. # = p < 0.1.
4. Discussion
In this study, we determined the effects of chronic methamphetamine exposure and Tat expression on activity and exploratory behavior in the behavioral pattern monitor (BPM). Utilizing both an established methamphetamine-exposure regimen (Kesby et al., 2018a; Kesby et al., 2019; Kesby et al., 2018b) and a validated mouse model of inducible Tat (iTat) expression (Langford et al., 2018), mice were assessed in the BPM 1) Before drug exposure (baseline BPM); 2) Shortly after chronic drug exposure, but before Tat induction with doxycycline (pre-DOX); and 3) After Tat induction following chronic drug exposure (post-DOX). At baseline, sex differences were observed wherein male mice exhibited higher activity and rearing-specific exploration but more linear activity as reflected by a lower spatial d. After chronic methamphetamine exposure (pre-DOX), the sex differences observed in baseline remained, while methamphetamine increased overall activity irrespective of sex, and decreased specific exploration and spatial d. After Tat induction (post-DOX), mice previously exposed to methamphetamine continued to exhibit elevated activity, but Tat expression did not affect activity, with no interaction observed. Following the post-DOX behavioral assessment, we observed modest decreases of DAT in the ventral tegmental area of methamphetamine-exposed mice, driven by saline-treated male wildtype mice.
Studies that utilize the BPM typically do not observe baseline sex differences in activity and exploration when tested in humans (Minassian et al., 2016; Perry et al., 2009), mice (Milienne-Petiot et al., 2017a; van Enkhuizen et al., 2014; van Enkhuizen et al., 2013; Young et al., 2010a, 2010b) or rats (Roberts et al., 2021). Here, we found that male mice displayed higher activity and exploration, and had simpler travelling paths than females, and these sex differences were maintained following chronic methamphetamine binge exposure. One other study from our group found male mice exhibited higher levels of activity and exploration, similar to our findings, but no changes in spatial d (Cope et al., 2021). The meaning of the sex differences are unknown but could be due to age, estrous cycle, or natural exploratory activity levels, each contributing to sex differences in activity using other paradigms e.g., the open field test (Datta et al., 2019; Simmel et al., 1976; Tran et al., 2021; van den Buuse et al., 2017). The BPM task more closely measures exploratory behavior and activity patterns however, and reduces potential predator fear relative to open field tests through the use of a closed lid apparatus. More work is therefore required to understand the meaning of any sex differences observed in the BPM.
Stimulant exposure alters each domain of motor function differently. For example, Torres et al. (2021) found methamphetamine exposed perinatal mice demonstrated hyper locomotor activity, but no changes to motor coordination as measured by the Rotarod test. Here, male and female mice demonstrated methamphetamine-induced hyperactivity, and decreased specific exploration and spatial d following a binge exposure. This pattern was consistent with acute amphetamine treatment in mice (Minassian et al., 2016; Perry et al., 2009; Young et al., 2010a). Additionally, animals continued to display altered activity and exploration following a week of methamphetamine abstinence, consistent with long-lasting stimulant-induced behavioral sensitization (Robinson and Becker, 1986; Valjent et al., 2010). These long-lasting behavioral effects are likely modulated by the dopaminergic system as methamphetamine increases dopamine activity by activation of dopamine-1 and −2 receptors (Brown et al., 2002; Sonsalla et al., 1986), increasing activity in animals (Camp et al., 1994; Pritchard et al., 2012). The increased activity and lower spatial d observed may be modulated by the DAT as genetic and pharmacological inhibition of DAT in mice caused the same pattern of behaviors (Milienne-Petiot et al., 2017a; Perry et al., 2009; van Enkhuizen et al., 2014; van Enkhuizen et al., 2013; Young et al., 2010a), though specific exploration is increased by these manipulations as seen in human bipolar patients, rather than decreased as demonstrated here and after amphetamine. Thus, the combined effect on DAT and norepinephrine inhibition likely underlies the change in specific exploration relative to selective DAT inhibition. The hyper-active and -exploratory phenotype of DAT KD mice was modulated by dopamine (Milienne-Petiot et al., 2017a; Milienne-Petiot et al., 2017b); thus, methamphetamine effects will likely have been inpart from inhibition of DATs.
Independently, both Tat and methamphetamine inhibit DAT function (Goodwin et al., 2009; Xie and Miller, 2009; Zhu et al., 2011; Zhu et al., 2009), and alter locomotor and exploratory responding (Henry et al., 2013; Henry et al., 2011; Nass et al., 2020). When combined, exposure to methamphetamine and Tat expression synergistically interacted to further dysregulate the dopaminergic system and dopamine-related behaviors (Cass et al., 2003; Liu et al., 2014; Maragos et al., 2002). For example, methamphetamine exposure in combination with intra-striatal Tat microinjections in rats produced significantly more dysregulation to striatal dopamine levels and DAT binding capacity, compared to methamphetamine or Tat alone (Maragos et al., 2002). At a behavioral level, Tat expression in mice produced increased methamphetamine-induced reward enhancement (Kesby et al., 2016), and locomotor sensitization (Kesby et al., 2017), and chronic methamphetamine further impaired PPI of these iTat mice (Walter et al., 2021b). Unexpectedly, the expression of Tat by DOX did not alter methamphetamine-induced alterations of activity or exploration in our study. The differences between our findings and those of Kesby et al. (2016, 2017) could be attributed to methamphetamine dosing regimens, as animals in their studies were exposed acutely to methamphetamine, rather than the chronic methamphetamine exposure in our study. The expression of Tat expression has increased (Joshi et al., 2020; Nass et al., 2020) and decreased (Liu et al., 2014; Walter et al., 2021b; Zhao et al., 2020) activity in rodents, however several factors vary across studies including the route (intracranial, systemic, food), and timing (acute, chronic) of DOX administration, and the particular apparatus used to monitor activity between laboratories (open field, homecage, BPM, novelty exploration, hole-board exploration), which may have driven these inconsistent findings. Indeed, within the same study, Joshi et al. (2020) report that acute DOX exposure by injection produced no activity changes in locomotor boxes, while chronic DOX exposure by food significantly decreased movement in the iTat mice. Some exploratory changes may occur over time with a long test session, but a within time-bin analysis over the 30 min did not reveal any findings that would suggest interaction between drug and genotype (data not shown).
Since Tat expression did not alter any BPM measures, the Tat protein likely does not synergistically nor additively contribute to the hyperlocomotor phenotype typically found in the BPM following stimulant exposure. In addition to altering behavior, the chronic methamphetamine binge exposure in our study produced subtle decreases of DAT in male mice. These findings are consistent with literature showing decreased DAT in human methamphetamine users using positron emission tomography (McCann et al., 1998; Sekine et al., 2001; Sekine et al., 2003; Volkow et al., 2001). These data are also consistent with methamphetamine-induced reductions of brain DAT in other rodent studies (Panmak et al., 2021). It should be noted that brains were collected following a 7 day washout period from the methamphetamine, and DAT expression can recover following drug abstinence (Volkow et al., 2001), therefore more robust decreases in DAT may have been observed across sex if brains were taken shortly following the methamphetamine exposure. Thus, methamphetamine-induced changes in BPM measures could relate to changes in brain DAT following stimulant exposure. The observed sex-differences could also mean that female DAT function recovers quicker than males.
In addition to the experimental limitations noted above, the iTat model in itself poses some constraints on the interpretation of our observed results. For one, the iTat model expresses one of the many HIV-1 relevant proteins (i.e. Tat) within a specific cell type (i.e. astrocytes; Kim et al., 2003; Langford et al., 2018). Thus, effects observed in this model are best understood as the specific impact of Tat-expressing astrocytes on physiological and cognitive functioning. However, while this model does not recreate the interaction of HIV-1 viral proteins found in PWH, it enables the investigation of Tat independently, helping to disentangle the effect of this specific protein on physiological and behavioral outcomes in HIV. Another caveat results from the use of doxycycline to induce Tat, as this compound has demonstrated neuroprotective properties in vivo and in vitro (see Santa-Cecilia et al., 2019 for a review), which may counteract Tat-induced neurotoxicity and relevant changes to physiology and behavior. It is therefore possible that doxycycline prevented an effect of Tat expression on BPM behavior in our study.
In conclusion, chronic binge methamphetamine exposure changed the locomotor activity, exploration, and movement patterns of mice in the BPM, consistent with previous stimulant literature. DOX-induced tat expression did not alter methamphetamine-induced changes, suggesting no effect or synergy of effects by this viral protein. Chronic binge methamphetamine exposure decreased DAT in the brains of male mice, possible contributing to the behavioral changes seen in the mouse BPM. Finally, these data indicate a lack of interactive effects by Tat and methamphetamine exposure on the basal locomotor activity in mice, suggesting that other viral proteins may contribute to such interactions, but also that studies on cognitive outcomes are unlikely to be confounded by changes in basal activity. These findings are important for future studies attempting to disentangle the impact of substance use on HAND-relevant behaviors using such transgenic animals.
Funding Acknowledgement
This work was supported by NIDA funding R01DA051295.
Data availability
Data will be made available on request.
References
- Appadoo CN, Sambo D, Alonge T, Harvey B, Khoshbouei H, 2017. The dual effect of HIV-1 Tat and methamphetamine on dopamine transporter function. FASEB J 31 (S1), 610–662. 10.1096/fasebj.31.1_supplement.662.10.28045376 [DOI] [Google Scholar]
- Baek EJ, Kim H, Basova LA, Rosander A, Kesby JP, Semenova S, Marcondes MCG, 2020. Sex differences and tat expression affect dopaminergic receptor expression and response to antioxidant treatment in methamphetamine-sensitized HIV tat transgenic mice. Neuropharmacology 178, 108245. 10.1016/j.neuropharm.2020.108245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B, 1994. Cerebrospinal fluid dopamine in HIV-1 infection. AIDS 8 (1), 67–71. 10.1097/00002030-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE, 2002. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J. Pharmacol. Exp. Ther 302 (2), 497–501. 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE, 1994. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus fischer 344 rats. Brain Res. 668 (1–2), 180–193. 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF, 2003. HIV-1 protein tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 984 (1–2), 133–142. 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS, 2008. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage 42 (2), 869–878. 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T, Marquez C, Hare CB, John MD, Klausner JD, 2012. Methamphetamine use, transmission risk behavior and internet use among HIV-infected patients in medical care, San Francisco, 2008. AIDS Behav. 16 (2), 396–403. 10.1007/s10461-010-9869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ZA, Kenton JA, Minassian A, Martin MV, Perry W, Bundgaard C, Arnt J, van Enkhuizen J, Geyer MA, Young JW, 2021. Chronic antipsychotic treatment exerts limited effects on the mania-like behavior of dopamine transporter knockdown mice. Behav. Brain Res 405, 113167 10.1016/j.bbr.2021.113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Samanta D, Tiwary B, Chaudhuri AG, Chakrabarti N, 2019. Sex and estrous cycle dependent changes in locomotor activity, anxiety and memory performance in aged mice after exposure of light at night. Behav. Brain Res 365, 198–209. 10.1016/j.bbr.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H, 2017. HIV, tat and dopamine transmission. Neurobiol. Dis 105, 51–73. 10.1016/j.nbd.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H, 2009. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem 284 (5), 2978–2989. 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W, Translational Methamphetamine ARCG, 2011. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology 215(4), 697–707. 10.1007/s00213-011-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A, Translational Methamphetamine, A.R.C.G., 2013. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav. Brain Res 236 (1), 210–220. 10.1016/j.bbr.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CR, Stacy S, Sumien N, Ghorpade A, Borgmann K, 2020. Astrocyte HIV-1 tat differentially modulates behavior and brain MMP/TIMP balance during short and prolonged induction in transgenic mice. Front. Neurol 11, 593188 10.3389/fneur.2020.593188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S, Translational Methamphetamine, A.R.C.G., 2015. Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. Eur. Neuropsychopharmacol 25 (1), 141–150. 10.1016/j.euroneuro.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S, 2016. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 109, 205–215. 10.1016/j.neuropharm.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Najera JA, Romoli B, Fang Y, Basova L, Birmingham A, Marcondes MCG, Dulcis D, Semenova S, 2017. HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function. Brain Behav. Immun 65, 210–221. 10.1016/j.bbi.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Chang A, Markou A, Semenova S, 2018. Modeling human methamphetamine use patterns in mice: chronic and binge methamphetamine exposure, reward function and neurochemistry. Addict. Biol 23 (1), 206–218. 10.1111/adb.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Fields JA, Chang A, Coban H, Achim CL, Semenova S, Group T, 2018. Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav Brain Res 349, 73–79. 10.1016/j.bbr.2018.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Chang A, Najera JA, Marcondes MCG, Semenova S, 2019. Brain reward function after chronic and binge methamphetamine regimens in mice expressing the HIV-1 TAT protein. Curr. HIV Res 17 (2), 126–133. 10.2174/1570162X17666190703165408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ, 2003. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol 162 (5), 1693–1707. 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski MA, Hellemann G, Sugar CA, Cope ZA, Minassian A, Perry W, Geyer MA, Young JW, 2019. Dopamine transporter knockdown mice in the behavioral pattern monitor: a robust, reproducible model for mania-relevant behaviors. Pharmacol. Biochem. Behav 178, 42–50. 10.1016/j.pbb.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Oh Kim B, Zou W, Fan Y, Rahimain P, Liu Y, He JJ, 2018. Doxycycline-inducible and astrocyte-specific HIV-1 tat transgenic mice (iTat) as an HIV/neuroAIDS model. J Neurovirol 24 (2), 168–179. 10.1007/S13365-017-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shi Z, Liu J, Wang Y, 2014. HIV transactivator of transcription enhances methamphetamine-induced Parkinson's-like behavior in the rats. Neuroreport 25 (11), 860–864. 10.1097/WNR.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA, 2002. Human immunodeficiency virus-1 tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J. Neurochem 83 (4), 955–963. 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA, 1998. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J. Neurosci 18 (20), 8417–8422. https://www.ncbi.nlm.nih.gov/pubmed/9763484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediouni S, Marcondes MC, Miller C, McLaughlin JP, Valente ST, 2015. The cross-talk of HIV-1 tat and methamphetamine in HIV-associated neurocognitive disorders. Front. Microbiol 6, 1164. 10.3389/fmicb.2015.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milienne-Petiot M, Geyer MA, Arnt J, Young JW, 2017. Brexpiprazole reduces hyperactivity, impulsivity, and risk-preference behavior in mice with dopamine transporter knockdown-a model of mania. Psychopharmacology 234 (6), 1017–1028. 10.1007/s00213-017-4543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milienne-Petiot M, Groenink L, Minassian A, Young JW, 2017. Blockade of dopamine D1-family receptors attenuates the mania-like hyperactive, risk-preferring, and high motivation behavioral profile of mice with low dopamine transporter levels. J. Psychopharmacol 31 (10), 1334–1346. 10.1177/0269881117731162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Young JW, Cope ZA, Henry BL, Geyer MA, Perry W, 2016. Amphetamine increases activity but not exploration in humans and mice. Psychopharmacology 233 (2), 225–233. 10.1007/s00213-015-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Morris SR, Kent CK, Stansell J, Klausner JD, 2006. Methamphetamine use and sexual activity among HIV-infected patients in care–San Francisco, 2004. AIDS Patient Care STDs 20 (7), 502–510. 10.1089/apc.2006.20.502. [DOI] [PubMed] [Google Scholar]
- Nass SR, Hahn YK, McLane VD, Varshneya NB, Damaj MI, Knapp PE, Hauser KF, 2020. Chronic HIV-1 tat exposure alters anterior cingulate cortico-basal ganglia-thalamocortical synaptic circuitry, associated behavioral control, and immune regulation in male mice. Brain Behav Immun Health 5. 10.1016/j.bbih.2020.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panmak P, Nopparat C, Permpoonpattana K, Namyen J, Govitrapong P, 2021. Melatonin protects against methamphetamine-induced Alzheimer's disease-like pathological changes in rat hippocampus. Neurochem. Int 148, 105121 10.1016/j.neuint.2021.105121. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, 1991. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 15 (6), 903–919. 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA, 2009. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch. Gen. Psychiatry 66 (10), 1072–1080. 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard LM, Hensleigh E, Lynch S, 2012. Altered locomotor and stereotyped responses to acute methamphetamine in adolescent, maternally separated rats. Psychopharmacology 223 (1), 27–35. 10.1007/s00213-012-2679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner BC, Keblesh JP, Xiong H, 2009. Methamphetamine abuse, HIV infection, and neurotoxicity. Int. J. Physiol. Pathophysiol. Pharmacol 1 (2), 162–179. https://www.ncbi.nlm.nih.gov/pubmed/20411028. [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA, 2006. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology 31 (11), 2349–2358. 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Roberts BZ, He YV, Chatha M, Minassian A, Grant I, Young JW, 2021. HIV transgenic rats demonstrate superior task acquisition and intact reversal learning in the within-session probabilistic reversal learning task. Cogn Affect Behav Neurosci 21 (6), 1207–1221. 10.3758/s13415-021-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, 1986. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 396 (2), 157–198. 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Santa-Cecilia FV, Leite CA, Del-Bel E, Raisman-Vozari R, 2019. The neuroprotective effect of doxycycline on neurodegenerative diseases. Neurotox. Res 35 (4), 981–986. 10.1007/s12640-019-00015-z. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N, 2001. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am. J. Psychiatry 158 (8), 1206–1214. 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N, 2003. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am. J. Psychiatry 160 (9), 1699–1701. 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Simmel EC, Haber SB, Harshfield G, 1976. Age, sex and genotype effects on stimulus exploration and locomotor activity in young mice. Exp. Aging Res 2 (3), 253–269. 10.1080/03610737608257180. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR, 1986. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. J. Pharmacol. Exp. Ther 238 (3), 932–937. https://www.ncbi.nlm.nih.gov/pubmed/2943891. [PubMed] [Google Scholar]
- Thaney VE, Sanchez AB, Fields JA, Minassian A, Young JW, Maung R, Kaul M, 2018. Transgenic mice expressing HIV-1 envelope protein gp120 in the brain as an animal model in neuroAIDS research. J Neurovirol 24 (2), 156–167. 10.1007/s13365-017-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres DJ, Yorgason JT, Andres MA, Bellinger FP, 2021. Methamphetamine exposure during development causes lasting changes to mesolimbic dopamine signaling in mice. Cell. Mol. Neurobiol 10.1007/s10571-021-01120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T, Mach J, Gemikonakli G, Wu H, Allore H, Howlett SE, Little CB, Hilmer SN, 2021. Male-female differences in the effects of age on performance measures recorded for 23 hours in mice. J. Gerontol. A Biol. Sci. Med. Sci 76 (12), 2141–2146. 10.1093/gerona/glab182. [DOI] [PubMed] [Google Scholar]
- UNAIDS, 2020. United Nations Programme on HIV/AIDS report. [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D, Girault JA, 2010. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 35 (2), 401–415. 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Buuse M, Low JK, Kwek P, Martin S, Gogos A, 2017. Selective enhancement of NMDA receptor-mediated locomotor hyperactivity by male sex hormones in mice. Psychopharmacology 234 (18), 2727–2735. 10.1007/s00213-017-4668-8. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW, 2013. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int. J. Neuropsychopharmacol 16 (5), 1021–1031. 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Halberstadt AL, Zhuang X, Young JW, 2014. Dopamine depletion attenuates some behavioral abnormalities in a hyperdopaminergic mouse model of bipolar disorder. J. Affect. Disord 155, 247–254. 10.1016/j.jad.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J, 2001. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci 21 (23), 9414–9418. https://www.ncbi.nlm.nih.gov/pubmed/11717374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim KM, Freedman EG, Tivarus ME, Heinecke A, Foxe JJ, 2022. Assessing combinatorial effects of HIV infection and former cocaine dependence on cognitive control processes: a functional neuroimaging study of response inhibition. Neuropharmacology 203, 108815. 10.1016/j.neuropharm.2021.108815. [DOI] [PubMed] [Google Scholar]
- Walter TJ, Iudicello J, Cookson DR, Franklin D, Tang B, Young JW, Perry W, Ellis R, Heaton RK, Grant I, Minassian A, Letendre S, On Behalf Of The Translational Methamphetamine Aids Research Center T., 2021. The relationships between HIV-1 infection, history of methamphetamine use disorder, and soluble biomarkers in blood and cerebrospinal fluid. Viruses 13 (7). 10.3390/V13071287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Young JW, Milienne-Petiot M, Deben DS, Heaton RK, Letendre S, Grelotti DJ, Perry W, Grant I, Minassian A, Translational Methamphetamine, A.R.C., 2021. Both HIV and tat expression decrease prepulse inhibition with further impairment by methamphetamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 106, 110089. 10.1016/j.pnpbp.2020.110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS, 2004. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127 (Pt 11), 2452–2458. 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu M, Lu Q, Farrell M, Lappin JM, Shi J, Lu L, Bao Y, 2020. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95 (19), e2610–e2621. 10.1212/WNL.0000000000010752. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM, 2009. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J. Pharmacol. Exp. Ther 330 (1), 316–325. 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA, 2010a. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology 208 (3), 443–454. 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA, 2010b. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol. Biochem. Behav 96 (1), 7–15. 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Fan Y, Vann PH, Wong JM, Sumien N, He JJ, 2020. Long-term HIV-1 tat expression in the brain led to neurobehavioral, pathological, and epigenetic changes reminiscent of accelerated aging. Aging Dis. 11 (1), 93–107. 10.14336/AD.2019.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM, 2009. HIV-1 tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H] dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J. Pharmacol. Exp. Ther 329 (3), 1071–1083. 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM, 2011. Recombinant human immunodeficiency virus-1 transactivator of transcription1-86 allosterically modulates dopamine transporter activity. Synapse 65 (11), 1251–1254. 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.