FIGURE 3.
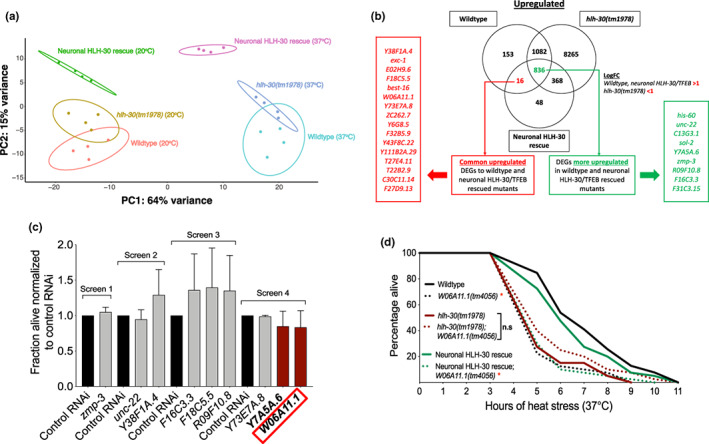
Neuronal HLH‐30 mediates thermoresistance through W06A11.1. (a) Principal component analysis (PCA) plot of wildtype, hlh‐30(tm1978), and neuronal HLH‐30/TFEB rescued animals based on the regularized log transformed gene count tables from the RNAseq analysis obtained from 20°C (control conditions) and 37°C (heat stress). (b) Genes upregulated by 37°C (heat stress) in comparison to 20°C (control conditions) for each genotype were overlapped to extract significant heat stress‐induced differentially expressed genes (adjusted p < 0.05) unique to or more upregulated (Log2 fold change [LogFC] thresholds applied as indicated) in wildtype and neuronal HLH‐30/TFEB rescued animals than hlh‐30(tm1978) mutants. (a and b) Animals from 4 independent replicates were developed at 20°C to day 1 of adulthood and harvested for RNA after 3 h of further growth at 20°C (control conditions) or 37°C (heat stress). (c) Neuronal HLH‐30/TFEB rescued animals were fed RNAi bacteria to knockdown heat stress‐upregulated genes for 48 h following development at 20°C to day 1 of adulthood, and scored for survival after 7 h of 37°C heat stress (n = 88–93/RNAi; fraction alive normalized to control RNAi per screen). Data are representative of 2 independent replicates. (d) Survival analyses of wildtype, hlh‐30(tm1978), and neuronal HLH‐30/TFEB rescued animals in the absence (solid lines) and presence (dotted lines) of W06A11.1(tm4056) loss of function at 37°C heat stress. Animals were developed at 20°C to day 1 of adulthood and exposed to 37°C heat stress until death. Data are representative of 2 independent replicates and comparisons were made by Mantel‐Cox log‐rank (n = 79–80/strain; n.s, p ≥ 0.05; *, p < 0.05; W06A11.1(tm4056) compared to control per genotype).
