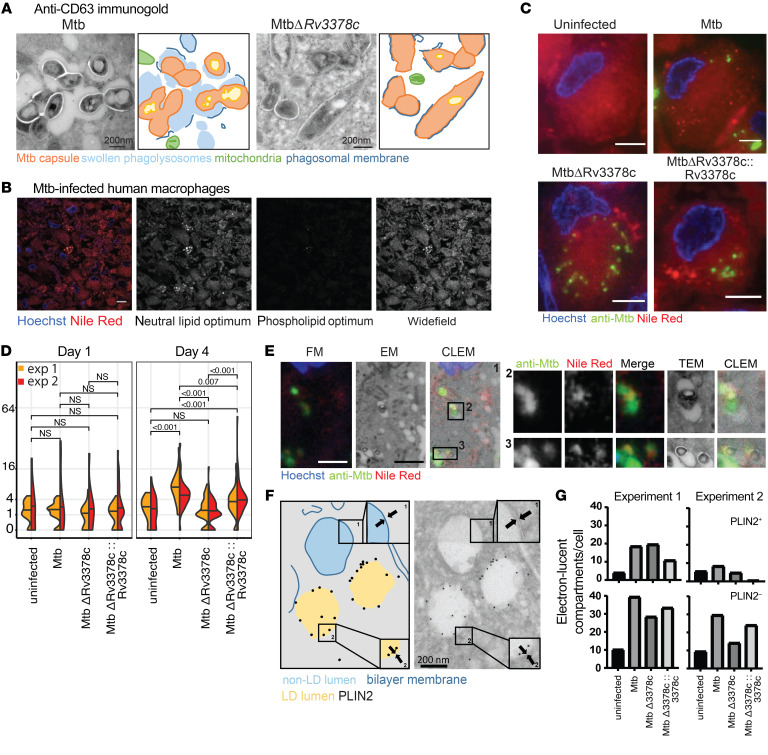
Figure 6. M. tuberculosis–produced 1-TbAd induces lipid accumulation in human macrophages.
(A) Human M1 macrophages were infected with M. tuberculosis or MtbΔRv3378c for 4 days, as reported previously (32), and were then subjected to anti-CD63 staining and annotated. Scale bars: 200 nm. (B) In a separate infection with WT M. tuberculosis, representative TEM images taken over 4 days showed lysosomal swelling. (C–E) Immunofluorescence images of human M1 macrophages infected for 4 days were stained with Hoechst (blue), anti–M. tuberculosis protein (green), and lipids with Nile red (red). The Nile red images were captured in excitation/emission detection windows that allowed broad detection of lipids (wide-field, 532–538 nm/570 nm), as well as detection of neutral lipids (515 nm/585 nm) and phospholipids (554 nm/638 nm). Wide-field Nile red puncta were quantified in 2 experiments with 35–56 cells for each infection condition. P values in panel D were determined by a least-squares means post test with adjustment by Tukey’s method after fitting a generalized linear mixed model and factorial ANOVA (overall P < 0.001 for strain). Data from 2 experiments were pooled after determining that the model fit was unchanged. In panel E, CLEM analysis of human macrophages infected for 4 days identified infected compartments and the limiting membranes of infected phagosomes with visible bacilli, along with staining for lipids (Nile red) and anti–M. tuberculosis antisera. Scale bars: 5 μm (B, C, and E). FM, fluorescence microscopy. (F and G) Human macrophages were infected with M. tuberculosis for 4 days, followed by staining with anti-PLIN2 immunogold. High-magnification images (insets 1 and 2) show a membrane bilayer and monolayer, respectively. In 2 independent experiments, 3,661 electron-lucent compartments stained with (PLIN2+) and without (PLIN2–) immunogold were counted in 9–17 cells per condition.