Figure 2.
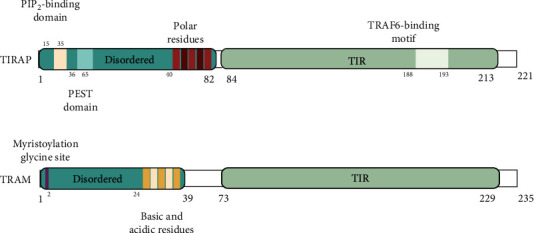
Structural view of human TIRAP and TRAM adaptor proteins. TIRAP contains a N-terminal PIP2-binding motif and a PEST domain, allowing polyubiquitination for rapid proteasomal degradation through suppressor of cytokine signaling 1 (SOCS1) binding [20]. TIRAP contains a C-terminal TIR domain. Its TRAF-6 binding motif permits direct association with TRAF6 for activation [21]. TRAM contains a N-terminal bipartite sorting signal that comprises its myristylation glycine site and controls its trafficking between the plasma membrane and the endosomes [22]. Similar to TIRAP, TRAM contains a C-terminal TIR domain.
