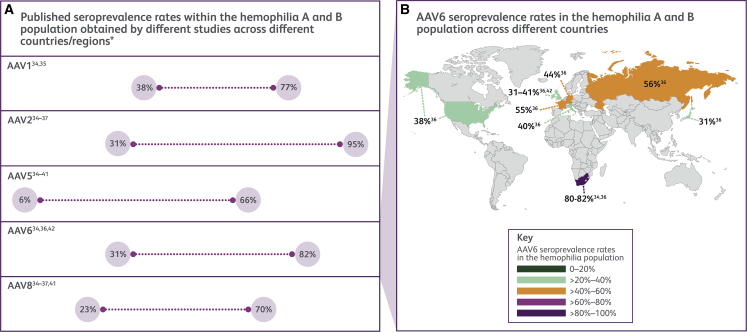
Figure 2.
Anti-AAV antibody seroprevalence ranges for different AAV serotypes and across geographical regions using the hemophilia A and B population as an example
The published anti-AAV antibody seroprevalence rates vary widely according to a variety of factors, including (A) different AAV serotypes,18,34,35,36,37,38,39,40,41,42 and (B) across geographical regions (using AAV6 data from three separate reports as an example).34,36,42 Other factors that may influence seroprevalence rates include assay type, donor health and age, and the use of some medications, such as immunosuppressants. ∗As these studies were performed in different regions/countries using different assay types, the seroprevalence rates cannot be directly compared. AAV, adeno-associated virus; TAb, total antibody; TI, transduction inhibition.