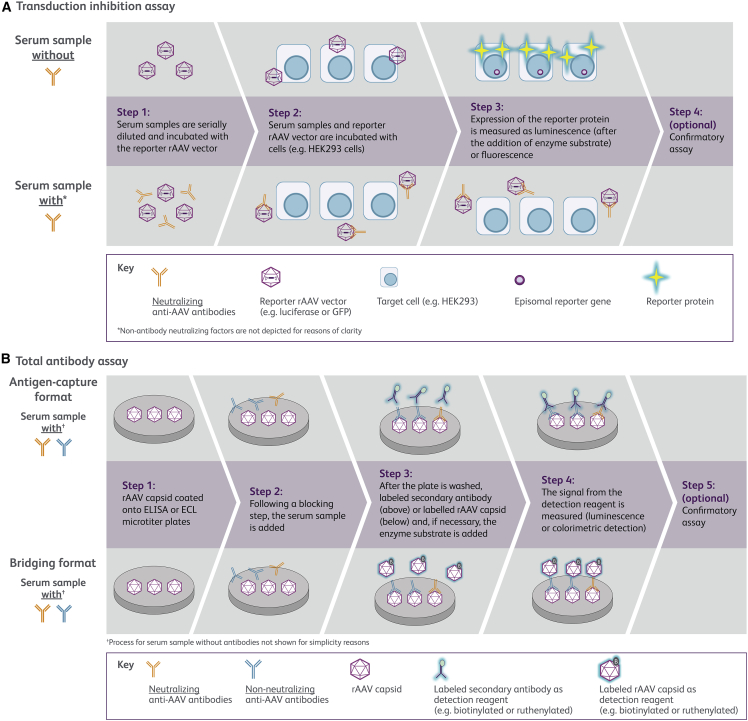
Figure 3.
Principles of transduction inhibition and total antibody assays
(A) (1) Serum samples are heat-inactivated and any potential precipitates removed by centrifugation. Patient and control serum dilutions are prepared. The TI assay is usually carried out in a 48- or 96-well plate format, permitting a high-throughput sample analysis. Typically, a reporter rAAV vector is combined with the serially diluted test sample before (2) being incubated with the cell line (in some cases, target cells are pre-infected with wild-type adenovirus to increase rAAV transduction).14 (3) The target cells are lysed, and reporter gene expression (luciferase or GFP activity) is measured as luminescence after the addition of the enzyme substrate (in the case of luciferase) or fluorescence (in the case of GFP). The presence of AAV NAbs and non-antibody neutralizing factors (not depicted in the figure for reasons of clarity) interferes with the transduction process and decreases the reporter gene expression when compared with the negative control. (4) Confirmatory steps to determine neutralization due to NAbs can be performed, although they are not always essential. This step may involve use of an irrelevant monoclonal non-AAV antibody to determine specificity, Ig fraction depletion, or competitive inhibition with empty vectors (or irrelevant transgenes).49 (B) TAb assays can be divided into antigen-capture and bridging formats, depending on the secondary reagents used. TAb assays can be developed using either co-incubation (homogeneous) or sequential incubation (heterogeneous) protocols based on the desired attributes, such as improved assay selectivity, antigen tolerance, or specificity (reviewed in Gorovits et al.48). (1) rAAV capsids are immobilized on an ELISA or electrochemiluminescence (ECL) microtiter plate; (2) serum samples are added to allow binding of antibodies to the rAAV capsid; (3) after washing to remove unbound material, in the antigen-capture format, a secondary detection reagent such as a horseradish peroxidase-conjugated or ruthenylated anti-species antibody is added; in the bridging format, a labeled (e.g., biotinylated or ruthenylated) rAAV capsid is added48). (4) In the case of a ruthenylated detection system, the assay signal is measured in luminescence units after the addition of the read buffer. For enzyme-conjugated detection systems, the enzyme substrate is added for colorimetric detection. Commercially available serotype-specific anti-AAV capsid monoclonal/polyclonal antibodies or proprietary antibodies may be used as a positive control, while pooled samples from TAb-negative donors can be used as a negative control.48 (5) Anti-AAV TAb screening assays typically involve an additional confirmatory assay to ensure the specificity of the signal obtained. AAV, adeno-associated virus; ECLA, electrochemiluminescence assay; ELISA, enzyme-linked immunosorbent assay; GFP, green fluorescent protein; Ig, immunoglobulin; NAb, neutralizing antibody; rAAV, recombinant AAV; TAb, total antibody; TI, transduction inhibition.