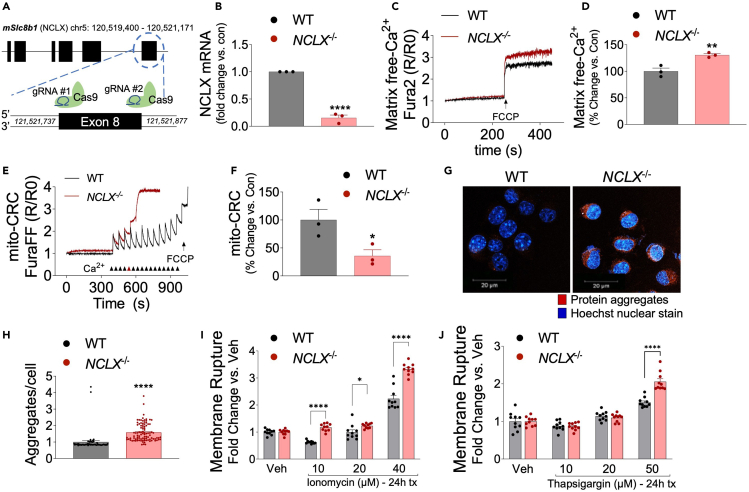
Figure 5.
Loss of NCLX increases mCa2+ overload, aggregate formation, and cell death
(A) Schematic for generation of NCLX knockout cell line (NCLX−/−) using CRISPR/SpCas9.
(B) NCLX mRNA expression in NCLX−/− and controls (WT) N2a cells, corrected to the housekeeping gene, Rps13; expressed as fold change versus control, n = 3 for both groups. All data presented as mean ± SEM; ∗∗∗∗p<0.001; two-tailed, unpaired t-test.
(C) Representative traces for basal mCa2+ content.
(D and E) (D) Quantification of mCa2+ content, n = 3 for both groups. All data presented as mean ± SEM; ∗∗p<0.001; two-tailed, unpaired t-test(E). Representative recordings of mCa2+ retention capacity.
(F and G) (F)Percent change in mCa2+ retention capacity versus N2a control cells, n = 3 for both groups. All data presented as mean ± SEM; ∗p<0.05; two-tailed, unpaired t-test(G). Representative images of intracellular protein aggregates in NCLX−/− and WT cells stained with Proteostat aggresome detection reagent (red) and Hoechst 33,342 nuclear stain (blue), scale bars = 20-μm.
(H) Total aggregates per cell, n = 96 for NCLX−/− and n = 58 for WT N2a control. All data presented as mean ± SEM; ∗∗∗∗p<0.001; two-tailed, unpaired t-test.
(I and J) NCLX−/− and WT cells were assessed for plasma membrane rupture, Sytox Green, after treatment with (I). Ionomycin (10–40 μM), (J). thapsigargin (10–50 μM), n = 10 for both groups. All data presented as mean ± SEM; ∗∗∗p<0.001, ∗∗p<0.01, ∗p<0.05; two-way ANOVA with Sidak’s multiple comparisons test.