Abstract
Introduction
Dysregulation of alternative complement pathway underlies the pathogenesis of both C3 glomerulopathy (C3G) and thrombotic microangiopathy (TMA). In this study, we describe both disease entities occurring in 5 patients.
Methods
We identified 114 patients at our institution from 2007 to 2016 with C3G in native kidney biopsies and those with concurrent TMA were included.
Results
The median age at diagnosis was 58 years (range: 28−69); all were male. Median serum creatinine and proteinuria at presentation were 2.3 mg/dl and 2089 mg/d, respectively. Three cases presented with TMA-predominant phenotype and 2 with C3G-predominant phenotype. Immunofluorescence (IF) showed bright C3 staining in mesangium and/or capillary walls. Electron microscopy showed marked subendothelial expansion by fluffy material in the capillary loops without associated deposits. However, capillary wall deposits were present in other loops in 4 cases. Mesangial deposits were present in all cases. Four cases showed low C3, of which 2 showed low C4. Complement evaluation in 3 cases showed pathogenic CFH mutation in 1 case, and multiple variant of unknown significance along with factor B autoantibody and C4 nephritic factor in 1 case. One patient negative for complement abnormalities had a monoclonal gammopathy. Three cases were treated with steroids and/or immunosuppressants. One case progressed to end-stage renal disease (ESRD) at 38.3 months; the remaining showed median serum creatinine and proteinuria of 2.5 mg/dl and 1169 mg/d, respectively at median follow-up of 17.5 months.
Conclusion
Overlap of C3G and TMA is rare and can clinically present as C3G-predominant or TMA-predominant phenotype. The significance of concurrent C3G/TMA findings on long-term renal survival remains to be explored.
Keywords: aHUS, alternative pathway of complement, complement factor H, complement-mediated TMA, C3 glomerulopathy, thrombotic microangiopathy
Dysregulation of alternative complement pathway resulting from acquired (e.g., autoantibodies) or genetic abnormalities (e.g., complement factor mutations) underlies the pathogenesis of both C3G and complement-mediated TMA.1, 2, 3, 4, 5, 6, 7 Therefore, although the underlying mechanisms may differ, the basic pathophysiology underlying both entities is dysregulation of alternative complement pathway. C3G and TMA have different clinical outcomes and prognosis.8, 9, 10, 11 The distinction between C3G and TMA is primary based on clinical, laboratory evaluation, and kidney biopsy findings.1,3,4
C3G is characterized by glomerular accumulation of complement proteins characterized by bright C3 staining (2+ or more) on IF microscopy with minimal or no staining for immunoglobulins.12 Based on ultrastructural findings, C3G is classified into 2 subtypes as follows: (i) C3 glomerulonephritis (C3GN) characterized by mesangial and capillary wall electron dense deposits and (ii) Dense Deposit Disease characterized by dense sausage-shaped osmiophilic intramembranous and mesangial deposits.12,13
TMA comprise a spectrum of disorders, including shiga toxin producing Escherichia coli-hemolytic uremic syndrome, complement-mediated TMA, thrombotic thrombocytopenic purpura, coagulation-mediated TMA, and other associated conditions. All these are linked by common pathophysiology ensuing from endothelial damage resulting in microvascular thrombosis.5,9 Complement-mediated TMA is also known as atypical hemolytic uremic syndrome and frequently results in kidney disease; and pathologic findings reflect tissue responses to endothelial injury characterized by endothelial swelling and mesangiolysis in active lesions, whereas double contouring of the glomerular basement membrane with subendothelial accumulation of fluffy material are more common in chronic lesions.14 All cases of TMA show no ultrastructural evidence of electron dense deposits unlike C3G, and IF is typically negative for bright C3 and immunoglobulins.11,14,15 Overt fibrin platelet thrombi may be absent, in which case the diagnosis is based on other morphologic features.11,14
Concurrent presence of C3G and TMA on kidney biopsies, to the best of our knowledge, has not been described previously. In this study, we describe the clinicopathologic features, including complement abnormalities, treatment, and outcomes in a series of 5 patients with an overlap of C3G and TMA.
Methods
On approval of our institutional review board, we identified 114 patients seen at the Mayo Clinic from 2007 to 2016 with a diagnosis of C3G (C3GN or Dense Deposit Disease) in native kidney biopsies and have characterized them extensively in our previous studies.10,16 On review of the kidney biopsy findings, 5 patients with histopathologic features of both C3G and TMA were identified. Review of medical records was then performed retrospectively, and details of clinicopathologic features, genetic abnormalities of complement pathway, and treatment outcomes were recorded. For analyzing 24-hour proteinuria at diagnosis and at follow-up, the average predicted proteinuria values (calculated based on urine protein-creatinine ratio) were considered for those patients in whom a quantitative 24-hour urine protein test was not conducted. Similar to our previously published studies,10,11 the estimated glomerular filtration rate (eGFR) at diagnosis and follow-up was calculated using the Chronic Kidney Disease Epidemiology Collaboration.17 Genetic studies were performed at an external institution as part of clinical evaluation and the methodologies utilized were similar to our prior studies.10
Results
Clinical Features and Laboratory Findings
All 5 cases were males, with a median age of 58 years (range: 28−69) at presentation. The median serum creatinine and proteinuria at initial diagnosis were 2.3 mg/dl (range: 2.0−3.2) and 2089 mg/24 hours (range: 250−5220), respectively. Four of 5 cases were White (80%), and one was unknown. All cases had a history of hypertension and were on antihypertensive medications, but none had a recent or active infection or autoimmune disease. The clinical and laboratory findings are detailed in Table 1.
Table 1.
Clinical and Laboratory Findings of patients with overlap of C3 glomerulopathy and thrombotic microangiopathy
| Data Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age at diagnosis (yr)/Sex | 69/Male | 28/Male | 67/Male | 58/Male | 33/Male |
| Hemoglobin at diagnosis (g/dl) | 10 | 9.7 | 7.4 | 7.5 | 9.6 |
| Schistocytes on peripheral blood smear | Present | Absent | Absent | NA | Absent |
| Platelet at diagnosis, 10(9)/l | 332 | 213 | 318 | 83 | 318 |
| ADAMTS13 | 93% | NA | NA | 41% | NA |
| Blood urea nitrogen at diagnosis (mg/dl) | NA | 28 | 44 | 27 | NA |
| Serum creatinine at diagnosis (mg/dl) | 2.3 | 2.2 | 2.8 | 3.2 | 2.0 |
| eGFR at diagnosis (ml/min/1.73 m2) | 28 | 39 | 22 | 20 | 43 |
| Serum albumin at diagnosis (g/dl) | NA | 4.2 | NA | 2.0 | NA |
| Proteinuria at diagnosis (mg/24 h) | 1500 | 5220 | 2089 | 250 | 3100 |
| Hematuria | Absent | Present, gross | Present, microscopic | Present, microscopic | Present, microscopic |
| C3 (mg/dl) | 49 | 74 | 87 | 64 | 64 |
| C4 (mg/dl) | NA | 10 | 40 | 6 | 27 |
| Complement pathway abnormalities |
CFH mutation (pathogenic) | CFAR1, CFHR5 and F2RL2 mutations (VUS), Factor B autoantibody, and C4 nephritic factor | NA | NA | Negative for mutation |
| Monoclonal Protein evaluation | Negative | Negativea | NA | NA | M-spike in gamma fraction, 0.3 g/dl |
| Treatment |
Recommended therapeutic plasma exchange (lost to follow-up at our institution)b | Steroid, mycophenolate mofetil | Conservative | Steroid | Steroid and therapeutic plasma exchange, eculizumab, cyclophosphamide |
| Progression to ESRD | No | No | No | No | Yes |
| Blood urea nitrogen at last lab workup (mg/dl) | 78 | 34 | NA | 29 | On dialysis |
| Serum creatinine at last lab workup (mg/dl) | 3.6 | 2.0 | 1.4 | 2.9 | On dialysis |
| eGFR at last lab workup (ml/min/1.73 m2) | 17 | 40 | 51 | 22 | On dialysis |
| Serum albumin at last lab workup (g/dl) | 4.8 | 3.2 | 4.2 | NA | On dialysis |
| Proteinuria at last lab workup (mg/24 h) | 2172 | 947 | 1169 | NA | On dialysis |
| Duration of follow-up from diagnosis to last clinical visit (mo)c | 18.9 | 26.5 | 4.0 | 16.0 | 50.3d |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; NA, not available/test not done; VUS, variant of unknown significance.
Reference ranges: Albumin: 3.5−5 g/dl; Blood urea nitrogen: 8−24 mg/dl; C3: 75−175 mg/dl; C4: 14−40 mg/dl
Patient had a type 3 cryoglobulinemia, but no monoclonal protein.
Patient was managed with antihypertensives to treat his increased blood pressure at the time of initial diagnosis.
Duration of follow-up refers to last known survival status of the patient.
Time to ESRD was 38.3 months from diagnosis, and final follow-up is 50.3 months from initial diagnosis.
A summary of each case is described below:
Patient 1
A 69-year-old male with history of prostate adenocarcinoma presented with symptoms of accelerated hypertension (systolic blood pressure ranging from 180−200 mm Hg and diastolic blood pressure about 100 mm Hg) and acute renal sufficiency with a rising serum creatinine of 2.3 mg/dl from a prior baseline of 1.5 mg/dl. He had a family history of ESRD of unknown etiology in his cousin. Evaluation at an outside facility revealed microangiopathic hemolytic anemia with hemoglobin of 10 g/dl, numerous schistocytes on peripheral blood smear, increased lactate dehydrogenase (297 U/l; reference: 120−250 U/) and low haptoglobin (<8 mg/dl, reference: 43−212 mg/dl). His ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) assay was within normal limits at 93% and he also had normal platelet count, thus ruling out thrombotic thrombocytopenic purpura. Initial kidney biopsy that was performed at the outside institution and re-reviewed at our institution, showed an overlap of subacute TMA and C3GN. At this point, he was managed on antihypertensive medications only. Approximately 18.9 months since initial diagnosis, the patient presented at our institution with increasing serum creatinine of 3.6 mg/dl, and proteinuria of 2172 mg/d. Autoimmune workup, including anticardiolipin antibody, lupus anticoagulant, antiphospholipid antibodies, antineutrophilic cytoplasmic antibodies, antinuclear antibodies, antidouble stranded-DNA antibodies (anti-double stranded-DNA) and anticentromere antibodies were negative. Serum protein electrophoresis was negative for a monoclonal protein. Genetic workup of the alternative complement pathway revealed a heterozygous pathogenic mutation in CFH gene (exon 16: c.2557T>C p.Cys853Arg), multiple genetic polymorphisms involving CFHR5 (heterozygous), CFB (homozygous), and CFH (heterozygous and homozygous) genes. C3 nephritic factor (C3Nef) and factor H autoantibodies were not detected, although he showed a low factor H level (143 mcg/ml, reference 160−412 mcg/ml) and low C3 (49 mg/dl; reference: 75−175 mg/dl). The remaining complement factors, including factor B, factor D, and factor I were within normal limits. He was recommended therapeutic plasmapheresis for a course of 2 weeks; however, he was subsequently lost to follow-up.
Patient 2
A 28-year-old male presented with microscopic hematuria for >5 years, bilateral swelling of lower extremities, abdominal pain, unintentional weight loss of >10% of body weight, anemia (hemoglobin: 9.7 g/dl) and renal insufficiency with serum creatinine of 2.2 mg/dl and proteinuria of 5220 mg/24 hours. At presentation, his blood pressure was 130/80 mm Hg. He had a history of type 3 cryoglobulinemia with positive hepatitis C virus antibodies (hepatitis C virus RNA not detected at current presentation), ulcerative colitis, and pulmonary non-Hodgkin’s lymphoma. On physical examination, he was found to have hepatosplenomegaly and bilateral micro-drusen on both eyes. Autoimmune workup, including antinuclear antibodies, anti-double stranded-DNA, antineutrophilic cytoplasmic antibodies, and anti-glomerular basement membrane antibodies were negative. Serum protein electrophoresis was negative for a monoclonal protein. Because of his decline in kidney function, a renal biopsy was done which revealed C3GN with ultrastructural evidence of subendothelial fluff consistent with features of chronic TMA. However, the patient had no schistocytes on peripheral blood smear and serum haptoglobin and serum lactate dehydrogenase were within normal limits. Further, genetic workup of the alternative complement pathway was negative for pathogenic mutations but showed multiple variants of unknown significance involving C5AR1 (NM_001736 c.559T>G,p.Leu187Val), CFHR5 (NM_030787 c.254-5C>T), and F2RL2 (NM_001256566 c.97G>A, p.Ala33Thr) genes, and heterozygous gene polymorphisms involving C3 and CFH. In addition, he tested positive for factor B autoantibody (1704 AU, reference <200 AU) and C4 nephritic factor (37.2% on immunofixation electrophoresis, reference <20%). He had borderline low C3 at 74 mg/dl (reference 75−175 mg/dl), and low C4 at 10 mg/dl (reference: 14−40 mg/dl). He was initiated on steroids and mycophenolate mofetil. At a follow-up of 26.5 months, he continued to have persistent microscopic hematuria with stable renal function with serum creatinine of 2 mg/dl, eGFR of 40 ml/min per 1.73 m2 and proteinuria of 947 mg/24 hours.
Patient 3
A 67-year-old male with history of JAK2 V617F mutation-positive essential thrombocythemia and ongoing treatment with hydroxyurea and lenalidomide presented with anemia (hemoglobin: 7.4 g/dl), leukocytosis (125.7 × 109/l; reference: 3.5−10.5 × 109/l), acute renal insufficiency with increase in serum creatinine from a baseline of 1.2 to 2.8 mg/dl. Urinalysis was positive for microscopic hematuria and a predicted 24-hour proteinuria of 2089 mg. He had no history of autoimmune diseases and had normal complement levels with C3 at 87 mg/dl and C4 at 40 mg/dl. His blood pressure at presentation was 151/64 mm Hg. A kidney biopsy revealed features of C3GN and ultrastructural evidence of TMA, and in addition showed involvement by patient’s known myeloproliferative neoplasm. Given his declining kidney function precipitated by his known myeloid neoplasm, lenalidomide was withheld, and he was commenced on ruxolitinib. His kidney function continued to improve with serum creatinine of 1.4 mg/dl, eGFR of 51 ml/min per 1.73 m2 and urinalysis showed a predicted 24-hour urine protein of 1169 mg/d. Comprehensive genetic evaluation of the alternative complement pathway was not performed. However, the patient died at 4.0 months from diagnosis and the cause of death was attributed to progressive myelofibrosis secondary to underlying essential thrombocythemia.
Patient 4
A 58-year-old male presented with bilateral lower extremity edema, anemia (hemoglobin: 7.5 mg/dl), chronic thrombocytopenia (83 × 10 (9)/l, reference 150–400 × 10(9)/l), low haptoglobin (21mg/dl, reference: 43−212 mg/dl) and renal insufficiency with serum creatinine at 3.2 mg/dl (baseline creatinine unknown) associated with microscopic hematuria and mild proteinuria of 250 mg/24 h. Autoimmune workup was negative for antineutrophilic cytoplasmic antibodies and antinuclear antibodies. His C3 and C4 were low at 64 mg/dl and 6 mg/dl, respectively. His ADAMTS13 was 41%, ruling out thrombotic thrombocytopenic purpura. His kidney biopsy was performed at an outside facility, which on further review at our institution confirmed C3GN and TMA. Blood pressure at the time of presenting symptoms is not available. Genetic evaluation of the alternative complement pathway was not performed. He was treated with steroids and subsequently his last laboratory workup showed a serum creatinine of 2.9 mg/dl, and calculated eGFR was 22 m/min per m2. He continues to follow-up at an outside facility.
Patient 5
A 33-year-old male with history of Kawasaki disease in childhood presented with pedal edema, chronic back pain, anemia, worsening renal function with serum creatinine of 2.0 mg/dl and nephrotic-range proteinuria of >3 g/d with hypoalbuminemia (quantitative serum albumin levels not available). His blood pressure was recorded in the range of 180 to 200 mm Hg systolic and 100 to 110 mm Hg diastolic. His family history was significant for kidney disease (of unknown etiology) in mother. He had no history of autoimmune disease. Monoclonal protein studies were positive for IgG λ with an M-spike of 0.3 g/dl. Light chain ratio was abnormal at 0.16 (reference: 0.26−1.65) with a serum free lambda light chain levels at 245 mg/l (reference: <26 mg/l). Renal biopsy showed features consistent with C3GN and TMA. Genetic workup of alternative pathways was negative for mutations. Complement levels of C3 was low at 64 mg/dl (reference 75−175 mg/dl), whereas C4 was normal at 27 mg/dl, respectively. Factor H level was reported to be low at an external institution (levels not available). He was initially treated at an outside facility with steroids, therapeutic plasma exchange, without substantial improvement. Later, he was initiated on eculizumab and given the presence of monoclonal protein, he also received cyclophosphamide. However, he continued to have worsening renal function, and at approximately 38.3 months from diagnosis, he progressed to ESRD and managed on hemodialysis biweekly or triweekly.
Kidney Biopsy Findings
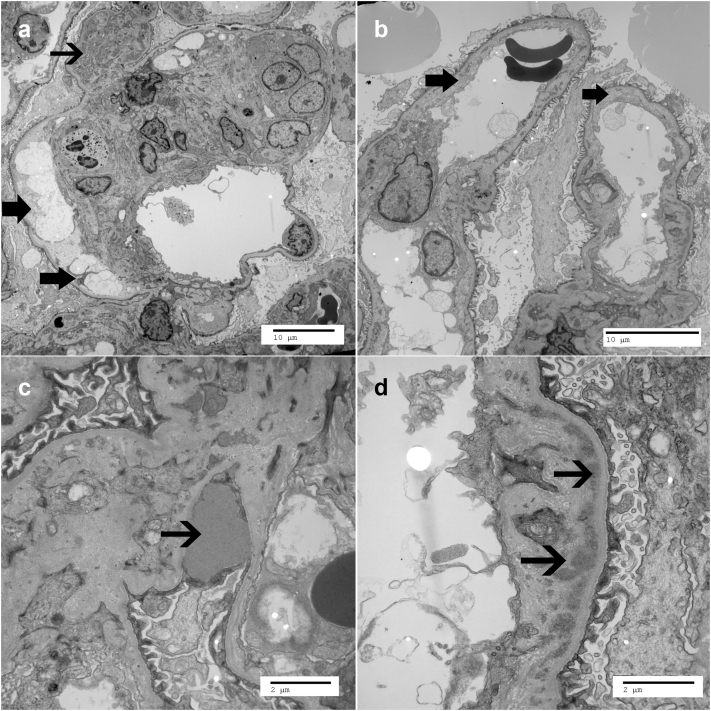
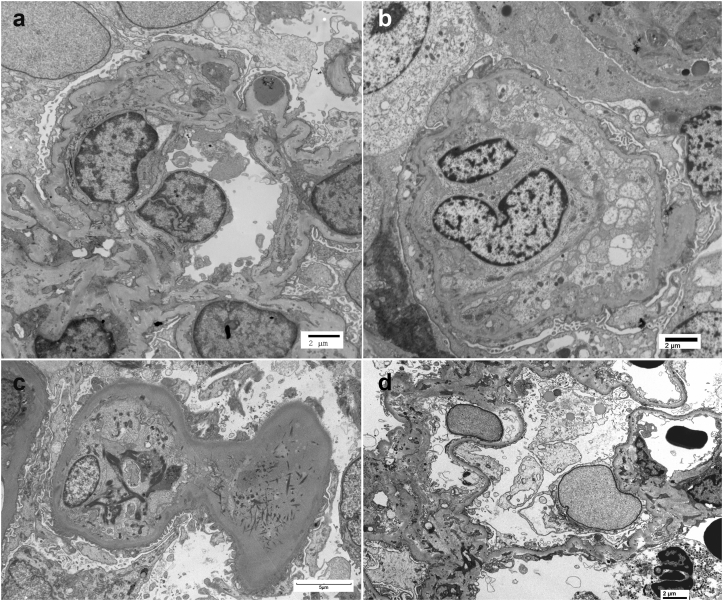
A summary of kidney biopsy findings are described in Table 2. Light microscopy showed an average of 16.2 glomeruli (SD: ±6.6), of which average sclerotic glomeruli was 16.3% (SD: ±16.2%). Three patients showed a membranoproliferative pattern of injury (Figure 1a), and remaining 2 showed mesangioproliferative pattern of injury. All except patient 1 showed mild interstitial fibrosis and tubular atrophy (10−25%, Table 2). IF showed bright C3 staining (≥2+, Figure 1b) in the mesangium and/or capillary walls with scant or negative immunoglobulins. On electron microscopy, capillary loops showed marked subendothelial expansion by fluffy electrolucent material (Figure 2a, and absence of deposits in these loops). All showed mesangial deposits (Figure 2a), 2 showed subepithelial humps (Figure 2c), and 4 showed capillary wall deposits (Figure 2d). Ultrastructural features of Dense Deposit Disease were not present; therefore, all biopsies were consistent with C3GN. It should also be pointed out that features of TMA were present in some loops, whereas other loops showed features of C3GN. In general, a TMA-dominant phenotype was present when greater than 25% of capillary loops showed ultrastructural features of TMA such as subendothelial fluff, endothelial swelling with compromise of capillary lumen, double contours in absence of electron dense deposits, and fibrin tactoids in the lumen (Figure 3). Three patients (1, 4, and 5) showed a TMA-dominant phenotype.
Table 2.
Kidney biopsy findings: summary of light microscopy, immunofluorescence, and electron microscopic findings
| Patient No. | Glomeruli: total/sclerosed | Pattern of injury | Thrombi in GC | Mesangiolysis | Double contours | Arteriosclerosis | %IFTA | IF | EM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10/2 | MPGN | Negative | + | + | 2+ | 30 | C3: 2+ | Mesangial and capillary wall deposits with humps, subendothelial fluff, double contours |
| 2 | 17/0 | MPGN | Negative | Absent | + | 1+ | 10 | C3: 3+; IgM: 1+ | Mesangial and capillary wall deposits, subendothelial fluff |
| 3 | 9/0 | MES | Negative | Present | - | 1+ | 20 | C3: 2+ | Mesangial and capillary wall deposits with humps, subendothelial fluff |
| 4 | 21/5 | MES | Negative | Absent | - | 1+ | 25 | C3: 3+ | Mesangial deposits and subendothelial fluff, endothelial swelling with capillary lumen compromise, double contours |
| 5 | 24/9 | MPGN/CRES with 40% crescents | Negative | Present | + | 2+ | 10 | C3: 3+ | Mesangial and capillary wall deposits, subendothelial fluff, endothelial swelling, double contours, fibrin tactoids |
CRES, crescentic; EM, electron microscopy; IF, Immunofluorescence; IFTA, interstitial fibrosis and tubular atrophy: grading: minimal (<10%); mild (10%−25%); moderate (26%−50%); severe (>50%), MPGN, membranoproliferative glomerulonephritis pattern of injury; MES, mesangial; SCL, sclerotic
Figure 1.
Representative Kidney biopsy findings: (a) Light microscopy showing membranoproliferative pattern of injury (silver methenamine 40×); (b) Immunofluorescence showing bright C3 in mesangium and along capillary walls.
Figure 2.
Representative electron microscopy findings of both C3GN and TMA. The thick arrows indicate (a) subendothelial fluff and (b) double contours; and thin arrow points to (a) mesangial deposits, (c) subepithelial hump and (d) capillary wall deposits.
Figure 3.
Additional electron microscopy findings showing features of TMA: (a) Ischemic loops with double contours (patient 1), (b) massive endothelial swelling and compromise of capillary lumen (patient 4), (c) endothelial swelling and fibrin tactoids (patient 5), and (d) mild subendothelial fluff (patient 2).
Clinical Follow-Up
Of the 5 cases, 1 progressed to ESRD at 38.3 months from initial diagnosis of C3G/TMA. For the remaining, at a median duration of 17.5 months (range: 4−26.5), the median serum creatinine, the median eGFR, and the median proteinuria were 2.5 mg/dl (range: 1.4−3.6 mg/dl), 31 ml/min per 1.73 m2 (range: 17−51 ml/min per 1.73 m2) and 1169 mg/24 hours (range: 947−2172, n = 3), respectively.
Discussion
In this short series of 5 patients, we show overlapping features of C3GN and TMA on the kidney biopsy. Although the kidney biopsy shows both C3GN and TMA findings, the clinical phenotype is either a TMA-predominant phenotype or a C3G-predominant phenotype.
In the appropriate setting, TMA is usually diagnosed by microangiopathic hemolytic anemia characterized by the presence of schistocytes on the peripheral blood smear and thrombocytopenia associated with ischemic organ damage such as acute kidney injury, neurologic abnormalities and/or cardiac ischemia.9,14 However, in cases of renal TMA, schistocytes may not be present on peripheral blood smear and therefore, diagnosis of TMA is made on histopathologic evaluation of kidney biopsies.18 On the other hand, C3G phenotype can vary from asymptomatic hematuria or proteinuria with preserved kidney function, to massive proteinuria or nephrotic syndrome, or a rapidly progressive glomerulonephritis.8
Of the 5 cases, 3 presented with a TMA-predominant clinical phenotype as follows: (i) patient 1 was characterized by microangiopathic hemolytic anemia, and acute renal insufficiency resulting from ischemic organ damage precipitated by malignant hypertension, (ii) patient 4 presented with hemolytic anemia (low haptoglobin) and thrombocytopenia, and (iii) patient 5 with history of Kawasaki disease, presented with anemia and massive proteinuria, and had an associated monoclonal gammopathy. All these 3 patients (1, 4, and 5) also showed a TMA-dominant phenotype on kidney biopsy (greater than 25% of capillary loops with ultrastructural features of TMA). In contrast to these TMA-predominant phenotypes, C3G-predominant clinical phenotype was observed in 2 patients as follows: (i) patient 2 presented with massive proteinuria, normal albumin, and without associated TMA clinical findings, and (ii) patient 3 with hematuria and subnephrotic proteinuria, although involvement by patient’s known myeloproliferative neoplasm was also the likely contributing factor to his declining kidney function.
Although none of the cases had associated triggers of C3G such as recent infections or autoimmune diseases,10 patient 1 and patient 3 showed subepithelial humps on electron microscopy but had no immunoglobulins on IF. These findings have been previously described in C3G with the hypothesis that the triggering event could still be a remote infection resulting in persistent regulatory dysfunction of the alternative pathway.15,19
Patient 5 is unique and deserves further mention. The patient had prior history of Kawasaki disease and subsequently was found to have a monoclonal gammopathy. Both C3G and TMA have been separately reported to occur in patients with monoclonal gammopathy.11,16 This is the first report to show concurrent C3GN and TMA in a patient with monoclonal gammopathy. Genetic studies were negative for complement pathway mutations, suggesting the monoclonal gammopathy was indirectly activating the complement pathway.11,16 In this case, given the reportedly low complement factor H-levels, we hypothesize that MIg acted like an autoantibody to CFH/complement regulating proteins, resulting in dysregulated alternative pathway. Both C3G and TMA associated with MIg show better renal survival when treated with targeted therapy for monoclonal protein and even though the patient was initiated on cyclophosphamide-based regimen, the patient did not receive clone-directed treatment.10,11 The history of Kawasaki disease, which is a systemic vasculitis, may have also involved the kidney resulting in vascular endothelial injury and could have potentially triggered the evolution of TMA.20 The patient’s kidney function continued to decline and given the complex past medical history, he eventually progressed to ESRD.
Genetic sequencing of complement genes was available in 3 of 5 cases, of which 2 were abnormal, with 1 case showing pathogenic CFH mutation, and the other with multiple variants of unknown significance along with factor B autoantibody and C4 nephritic factor. Genetic variants in CFH gene are more common in both TMA and C3G.21,22 In TMA, typically CFH mutations are missense variants that alter the C3b-and polyanion-binding site at the C-terminus of factor H, resulting in decreased plasma factor H-levels and increased susceptibility of cells to complement lysis. In contrast, C3G related mutations in CFH involve the N-terminus resulting in persistent activation of complement in plasma, causing deposition of complement products in the glomerular basement membrane.21,23 In patient 1, it is possible that the mutation in exon 16 of CFH gene resulted in low factor H-levels triggering the pathogenesis of TMA and C3G.24,25 An interesting finding in patient 2 was the presence of a CFHR5 genetic variant, which contain a shared dimerization motif that confers avidity for tissue-bound complement fragments that compete with the physiological complement inhibitor, complement factor H (CFH), thereby predisposing to disease.26,27 Further, CFHR5 also shares some of the complement regulatory activities with CFH that also underlies the pathogenesis of drusen formation (as seen in this patient) in the setting of C3G.28, 29, 30 Drusen is characterized by lipoproteinaceous deposition of products of the alternative complement pathway, which is present between the retinal pigment endothelium and the Bruch’s membrane.28
In summary, our series is the first to the best of our knowledge detailing the clinical course of patients with an overlap of C3G and TMA syndrome. Abnormalities in alternative complement pathways can clinically result in either a C3G-predominant phenotype or TMA-predominant phenotype, with kidney biopsy showing features of both C3G and TMA. Our findings signify the importance of documenting the presence of concurrent C3G or TMA findings on kidney biopsy and whether these have long-term impact on renal survival remains to be explored.
Disclosure
All the authors declared no competing interests.
Author Contributions
AR and SS designed the study, collected the clinical data, reviewed the biopsies, and acquired the pathology images. LMP and FCF reviewed and confirmed the accuracy of clinical data. All authors (AR, LMP, FCF, and SS) critically reviewed and contributed to the contents of this manuscript.
References
- 1.Goodship T.H., Cook H.T., Fakhouri F., et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Cserhalmi M., Uzonyi B., Merle N.S., et al. Functional characterization of the disease-associated N-terminal complement factor H mutation W198R. Front Immunol. 2017;8:1800. doi: 10.3389/fimmu.2017.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vriese A.S., Sethi S., Van Praet J., et al. Kidney disease caused by dysregulation of the complement alternative pathway: an etiologic approach. J Am Soc Nephrol. 2015;26:2917–2929. doi: 10.1681/ASN.2015020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurman J.M. Complement in kidney disease: core curriculum 2015. Am J Kidney Dis. 2015;65:156–168. doi: 10.1053/j.ajkd.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma L.M.P., Sridharan M., Sethi S. Complement in secondary thrombotic microangiopathy. Kidney Int Rep. 2021;6:11–23. doi: 10.1016/j.ekir.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma L.M.P., Vaisbich-Guimarães M.H., Sridharan M., et al. Thrombotic microangiopathy in children. Pediatr Nephrol. 2022;37:1–14. doi: 10.1007/s00467-021-05370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Józsi M., Reuter S., Nozal P., et al. Autoantibodies to complement components in C3 glomerulopathy and atypical hemolytic uremic syndrome. Immunol Lett. 2014;160:163–171. doi: 10.1016/j.imlet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Bomback A.S., Santoriello D., Avasare R.S., et al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018;93:977–985. doi: 10.1016/j.kint.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Go R.S., Winters J.L., Leung N., et al. Thrombotic microangiopathy care pathway: a consensus statement for the Mayo Clinic complement alternative pathway-thrombotic microangiopathy (CAP-TMA) disease-oriented group. Mayo Clin Proc. 2016;91:1189–1211. doi: 10.1016/j.mayocp.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Ravindran A., Fervenza F.C., Smith R.J.H., et al. C3 glomerulopathy: ten years’ experience at Mayo Clinic. Mayo Clin Proc. 2018;93:991–1008. doi: 10.1016/j.mayocp.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravindran A., Go R.S., Fervenza F.C., Sethi S. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017;91:691–698. doi: 10.1016/j.kint.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 12.Pickering M.C., D’Agati V.D., Nester C.M., et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S., Vrana J.A., Fervenza F.C., et al. Characterization of C3 in C3 glomerulopathy. Nephrol Dial Transplant. 2017;32:459–465. doi: 10.1093/ndt/gfw290. [DOI] [PubMed] [Google Scholar]
- 14.Brocklebank V., Wood K.M., Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol. 2018;13:300–317. doi: 10.2215/CJN.00620117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi S., Fervenza F.C. Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS) Semin Thromb Hemost. 2014;40:416–421. doi: 10.1055/s-0034-1375701. [DOI] [PubMed] [Google Scholar]
- 16.Ravindran A., Fervenza F.C., Smith R.J.H., Sethi S. C3 glomerulopathy associated with monoclonal Ig is a distinct subtype. Kidney Int. 2018;94:178–186. doi: 10.1016/j.kint.2018.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CKD-EPI adults (conventional units). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate-calculators/ckd-epi-adults-conventional-units
- 18.Murphree C.R., Nguyen N.N., Shatzel J.J., et al. Biopsy-proven thrombotic microangiopathy without schistocytosis on peripheral blood smear: a cautionary tale. Am J Hematol. 2019;94:E234–E237. doi: 10.1002/ajh.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi S., Fervenza F.C., Zhang Y., et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83:293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T., Watanabe T. Kidney and urinary tract involvement in Kawasaki disease. Int J Pediatr. 2013;2013 doi: 10.1155/2013/831834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merinero H.M., Garcia S.P., Garcia-Fernandez J., et al. Complete functional characterization of disease-associated genetic variants in the complement factor H gene. Kidney Int. 2018;93:470–481. doi: 10.1016/j.kint.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Pickering M.C., de Jorge E.G., Martinez-Barricarte R., et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204:1249–1256. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragon-Durey M.A., Fremeaux-Bacchi V., Loirat C., et al. Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- 24.Pickering M.C., Cook H.T. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernon K.A., Ruseva M.M., Cook H.T., et al. Partial complement factor H deficiency associates with C3 glomerulopathy and thrombotic microangiopathy. J Am Soc Nephrol. 2016;27:1334–1342. doi: 10.1681/ASN.2015030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goicoechea de Jorge E., Caesar J.J., Malik T.H., et al. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A. 2013;110:4685–4690. doi: 10.1073/pnas.1219260110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vernon K.A., Gale D.P., de Jorge E.G., et al. Recurrence of complement factor H-related protein 5 nephropathy in a renal transplant. Am J Transplant. 2011;11:152–155. doi: 10.1111/j.1600-6143.2010.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savige J., Amos L., Ierino F., et al. Retinal disease in the C3 glomerulopathies and the risk of impaired vision. Ophthal Genet. 2016;37:369–376. doi: 10.3109/13816810.2015.1101777. [DOI] [PubMed] [Google Scholar]
- 29.Gale D.P., de Jorge E.G., Cook H.T., et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gale D.P., Pickering M.C. Regulating complement in the kidney: insights from CFHR5 nephropathy. Dis Model Mech. 2011;4:721–726. doi: 10.1242/dmm.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]