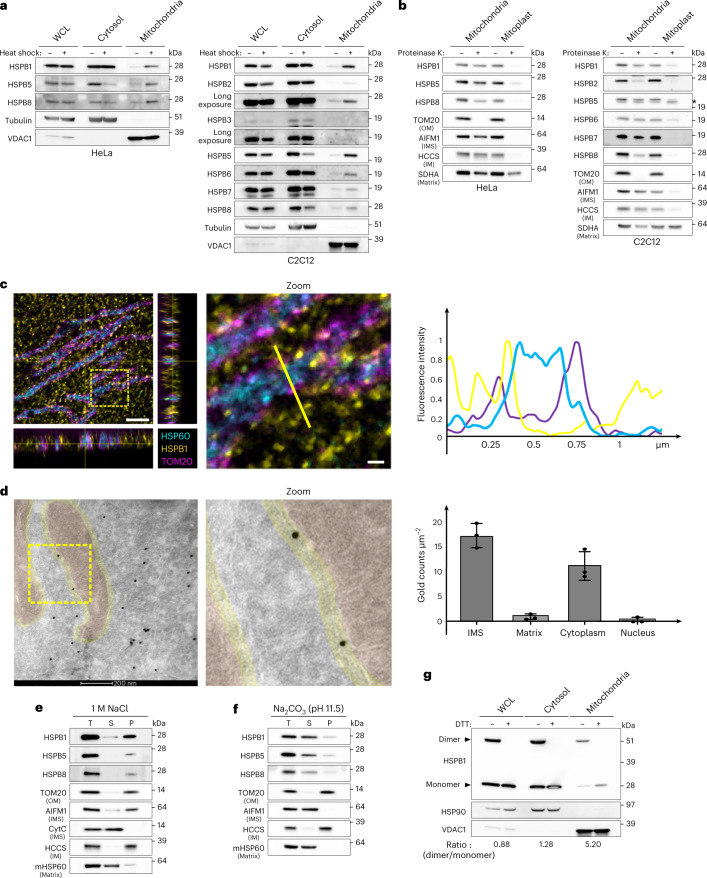
Fig. 1. The mitochondrial IMS contains sHSPs.
a, HeLa and C2C12 cells were subjected to heat shock (42 °C) for 1 h. The mitochondrial fraction was separated from the cytosolic fraction, and the abundance of different sHSPs in each fraction was verified by immunoblotting. Tubulin was used as a marker for the cytosol and VDAC1 for mitochondria. Whole cell lysate (WCL) represents the NP-40 soluble fraction and was used as a reference. b, Submitochondrial localization of sHSPs was verified by subjecting intact mitochondria and mitoplasts (derived after osmotic swelling) to proteinase K (10 μg ml−1) treatment. Matrix, mitochondrial matrix; *, lowest band is non-specific. c, Expansion microscopy of non-heat-shocked HeLa cells with immunofluorescence staining of endogenous HSPB1, HSP60 (matrix) and TOM20 (OM). Samples were fixed and stained before linkage in an expandable hydrogel, and imaged with confocal laser scanning microscopy. Zoomed area (3 µm × 3 µm) and line plot are represented. Indicated distances are corrected for the expansion factor (4×). Scale bar, 2.0 μm and 0.25 μm (zoom). d, Representative electron micrograph of non-heat-shocked HeLa cells transduced with HSPB1-GFP and stained for anti-GFP. In three independent electron microscopy samples, 554–814 gold particles were scored on 14–16 images per sample and corrected for area. Local density estimations are presented (mean ± s.d.). Scale bar, 200 nm. e,f, Mitochondria were isolated from HeLa cells and treated with 1 M NaCl (e) or Na2CO3 at pH 11.5 (f). T, total; P, pellet; S, supernatant. g, Mitochondria, cytosolic fraction and WCL from HeLa cells were boiled in sample buffer with or without DTT to destroy or maintain disulphide bonds, respectively. The ratio of monomers to dimers was calculated by densitometry and corrected for loading with VDAC1. Results are representative of two (c) or three replicates (a, b, d, e, f and g). Source numerical data and unprocessed blots are available in source data.