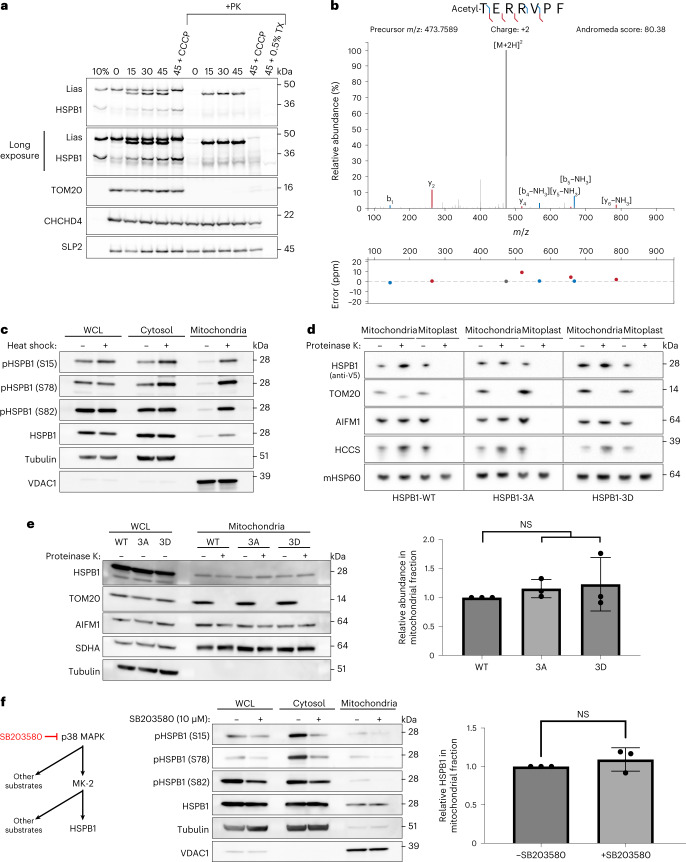
Fig. 2. Mitochondrial import of sHSPs is not dependent on phosphorylation or mitochondrial membrane potential.
a, Recombinant [35S]-labelled LIAS and HSPB1 were produced with rabbit reticulocyte lysate and incubated with mitochondria purified from Hela cells for the indicated times (min) in the presence or absence of a membrane potential (+CCCP) across the IM. Treatment with proteinase K (PK) led to the degradation of non-imported proteins, while treatment with Triton X-100 (TX) allowed the digestion of all soluble proteins, controlling for protease resistance due to protein aggregation. Samples were analysed by SDS–PAGE followed by autoradiography or immunoblotting. TOM20 is a marker for the OM, CHCHD4 for the IMS and SLP2 for the IM. 10% represents the input fraction of recombinantly produced proteins. b, HSPB1 was immuno-precipitated from purified mitochondria using the V5-tag, after treatment with proteinase K to digest all accessible (non-imported) HSPB1. Bands were cut from a Coomassie-stained gel and analysed by LC–MS/MS, which revealed the removal of the N-terminal methionine and the addition of acetyl to the N-terminal peptide. c, HeLa cells were subjected to heat shock for 1 h. Phosphorylation of HSPB1 was determined with phospho-specific antibodies directed towards Ser15, Ser78 and Ser82. d, Submitochondrial localization of sHSPs was verified by subjecting intact mitochondria and mitoplasts (derived after osmotic swelling) to proteinase K (10 μg ml−1) treatment. e, Phosphorylation variants were transfected in HeLa cells and analysed for their mitochondrial import. Substitution of all three phosphorylation residues with alanine (HSPB13A) or aspartate (HSPB13D) represents non-phosphorylated and phosphorylated HSPB1, respectively. Proteinase K (10 μg ml−1) was added to verify if the proteins were imported. Densitometric analysis of the bands is represented (mean ± s.d.) (n = 3 biologically independent experiments). One-way ANOVA with Dunnett’s multiple comparison test was performed. NS, non-significant. f, Inhibition of the MAPK pathway with SB203580 (10 μM) in HeLa cells. Phosphorylation of HSPB1 was determined by phospho-specific antibodies. Densitometric analysis was performed for the mitochondrial fraction and presented in the graph (mean ± s.d.) (n = 3 biologically independent experiments). Two-tailed unpaired Student’s t-test was performed. Results are representative of two (a) or three replicates (c, d, e and f). Source numerical data, including exact P values, and unprocessed blots are available in source data.