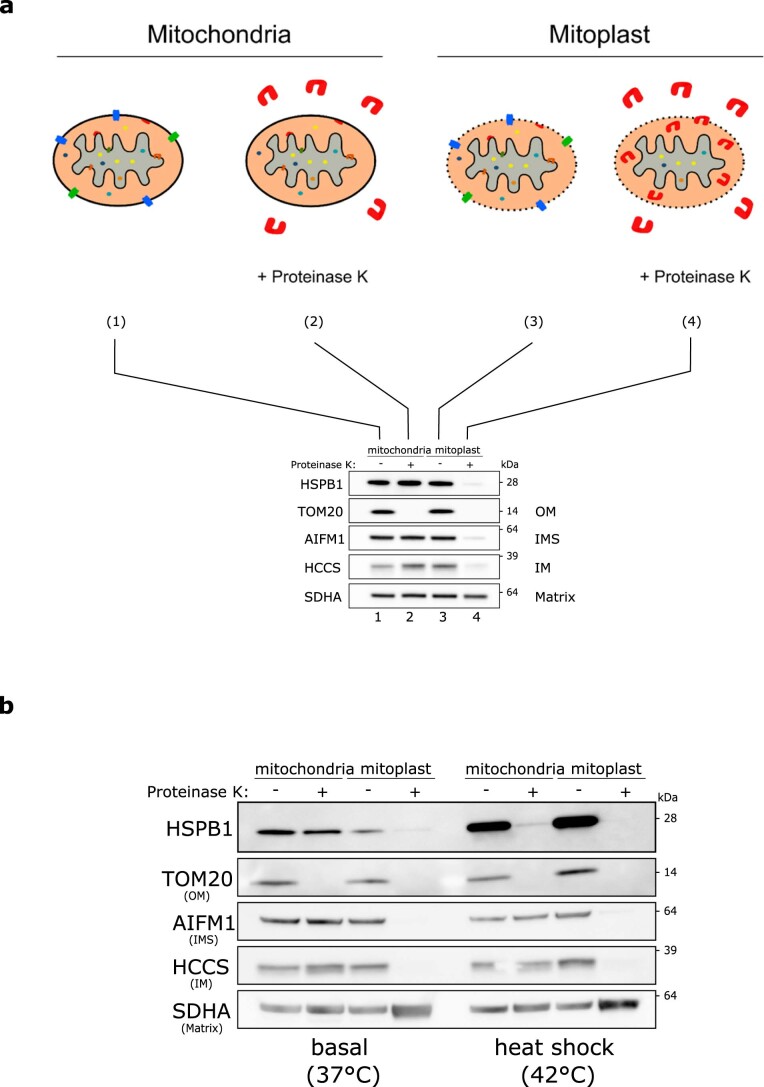
Extended Data Fig. 2. Small heat shock proteins reside in the mitochondrial intermembrane space under basal conditions and accumulate at the outer membrane after heat shock.
a, Scheme of the proteinase K protection assay. Mitochondria were isolated from HEK293T cells and divided over 4 different tubes. The first two tubes contained regular mitochondrial isolation buffer (1 + 2) while the last two tubes (3 + 4) contained mitochondrial swelling buffer. Due to the absence of sucrose/mannitol in the swelling buffer, mitochondria were subjected to osmotic swelling which causes rupture of the OM (but not the IM) leading to mitoplast formation. The addition of proteinase K to one tube of each (2 and 4) resulted in the digestion of all accessible proteins. For (2) this leads to the digestion of all non-imported/OM proteins, for (4) this leads to the digestion of all OM, IMS, and IMS-facing IM proteins. Matrix proteins remain protected by the intact IM. The western blot displays the end result with markers for each of the different mitochondrial compartments. b, Proteinase K protection assay on mitochondria isolated from HeLa cells before and after heat shock. Isolated mitochondria were resuspended in a hypotonic (osmotic swelling) buffer to generate mitoplasts. Proteinase K (10 μg/ml) was used to digest accessible proteins. Samples were analyzed by SDS-PAGE followed by immunoblotting using anti-HSPB1, anti-TOM20 (OM), anti-AIFM1 (IMS), anti-HCCS (IM), and anti-SDHA (matrix) antibodies. Note that compared to Fig. 1 more mitochondrial protein was loaded, without WCL and cytosolic fractions on the same gel, resulting in higher detection sensitivity. Results in (b) are representative of five replicates. Unprocessed blots are available in source data.