Abstract
Evidently proven medicinal benefits of Tinospora cordifolia and the growing demand of functional foods have created scientific interest in the functional beverage. Therefore, an attempt was made to prepare probiotic Lactiplantibacillus pentosus GSSK2 supplemented herbal wine having the benefits of both phytochemical and probiotic. Experimentally, fermentation of Tinospora cordifolia stem was found to be the most effective with ammonium dihydrogen phosphate, potassium phosphate, magnesium sulfate, isoleucine, and thiamine that yielded maximum ethanol (6.8 to 10%), total phenol (419 to 791.5 µg/ml), and antioxidants capacity (98.2 to 160.4 µmol/ml) after optimizing physical parameters, i.e., 20° Brix total soluble solid, pH 4.5, temperature 30 °C, and 10% (v/v) inoculum. Further, prepared herbal wine was supplemented separately with seven different probiotic strains and among these Lactiplantibacillus pentosus GSSK2 had the highest 88.6% survival rate compared with other probiotics and was safe showing 100% survivability of HEK-293 and THP-1 cells. Both herbal- and probiotic-supplemented herbal wine showed the antimicrobial potential against Gram-positive and Gram-negative bacteria as probiotic-supplemented herbal wine had 19–21 mm inhibition zone compared with 18–19 mm with herbal wine. LC–MS analysis of the probiotic-supplemented herbal wine revealed the presence of various phytochemicals such as alkaloids, diterpenoid lactone, glycoside, steroids having anti-bacterial, anti-oxidant, and anti-inflammatory potential. This is the first ever such study to demonstrate the antibacterial, antioxidant potential and safety of probiotic supplemented herbal wine in vitro.
Keywords: Antioxidant, Ethanol, Functional foods, Phenolics, Probiotics, Tinospora cordifolia (giloy), Wine
Introduction
The increasing awareness toward the health and nutrition had led to the explosion of consumers’ interest in functional foods. Functional foods are defined as products which contain various biologically active compounds and are consumed as a part of the usual diet with demonstrated physiological benefits and/or reduce the risk of chronic disease beyond basic nutritional functions (FAO 2004).
Functional foods are prepared with bioactive compounds either of plant/animals’ origin or even some bacterial strain, i.e., probiotics and such functional foods modulate different biological activities (Mitsuoka 2014; Mirmiran et al. 2014). At present, beverages are the most active functional food category due to their excellent delivering means for nutrients and bioactive compounds such as vitamins, minerals, antioxidants, plant extracts, and probiotics (Corbo et al. 2014). Interestingly, in today’s world, beverages are no longer considered simply as thirst quenchers; instead, consumers look for specific functionality in beverages, which forms a part of their lifestyle, i.e., dairy beverage (Yakult, Vitagen), vegetable and fruit beverage, e.g., Goodbelly, daily greens (Ozer and Kirmaci 2010).
Probiotics are defined as live bacteria when administrated in adequate amount confer the benefit to the host and the most widely studied and “generally regarded as safe” (GRAS) probiotic strains include species from the genera Lactobacillus and Bifidobacterium (FAO/WHO 2002). Moreover, probiotics owe their recently gained attention from both health and food industry to their multifactorial potentials such as, anti-colon cancer effect, aid in lactose digestion, immune system modulation, blood lipid, heart disease, resistance to enteric pathogen, antihypertensive effect, metabolic disorders, and urogenital infection (Mallappa et al. 2012; Verma and Shukla 2013). Functional beverages, particularly supplemented with probiotic (e.g., Boza, Togwa, Bushera) have recently gained more attention because of their beneficial effects such as immune-stimulation, reduces intestinal dysbiosis, alleviation of oxidative stress, cancer, diabetes, cholesterolemia (Maldonado et al. 2017; Slima et al. 2018). Although, dairy fermented products such as yogurts, cheese, and fermented sour milk have been traditionally considered as the best probiotic carriers, due to increasing number of lactose-intolerant individuals and adverse effects of cholesterol containing probiotic, dairy beverages emphasizes the need for novel dairy free beverages (Vasudha et al. 2013).
Among various non-dairy beverages, wine is the most validated and researched beverages due to its health benefits particularly when consumed in moderate quantity on regular basis. Generally, wine is prepared from grapes, but now a days, herbs are also emerging as a better choice for making improved wine in terms of its functionality (Chauhan et al. 2015; Swami et al. 2016). In the past, various herbs such as aloe vera, amla, ginger, peppermint have been used to prepare herbal wine (Soni et al. 2009; Tiwari et al. 2017). The perceived medicinal value associated with these herbs gets imparted into the wines prepared from them and the bioactive compounds such as alkaloids, glycoside, phenolics of the herbs add to their antimicrobial, anti-cancerous, and antioxidant activity that can play important role in either preventing or treating non-communicable disease such as diabetes, coronary disease, cardiovascular disease (Swami et al. 2017; Bhise and Morya 2021). In this context, Tinospora cordifolia (Giloy), the most prevailing genera in the family Menispermaceae, an important drug of Indian Systems of Medicine is a common deciduous plant growing over hedges and small tress (Sinha et al. 2004). Tinospora genus contains around 15 distinct species and is scattered in tropical region of India upto 1200 m above sea level from Kumaon to Assam, in north extending through West Bengal, Kerala, Bihar, and reaching an altitude of 1000 ft in Indonesia, Thailand, Myanmar, China, and Srilanka worldwide (Wet et al. 2008; Modi et al. 2021). As various parts of giloy have been found to contain numerous compounds such as steroids, alkaloids, polysaccharides, aliphatic compound, terpenoids due to which it has a diverse medicinal potential such as anti-cancerous, anti-inflammatory, anti-diabetic, antioxidant activity, anti-ulcer activity, hypolipidaemic activity, and liver disorders (Upadhaya et al. 2010; Gupta et al. 2011).
To the best of our knowledge, no information pertaining to the preparation, optimization of herbal wine from giloy, and its supplementation with probiotic and safety analysis is available and warrants further study. More specifically, with the growing global demand for functional beverages particularly with probiotics, the present study was designed to prepare, optimize novel and versatile functional beverage having benefit of both phytochemical (Tinospora cordifolia) and probiotic (probiotic supplemented herbal wine) vis-à-vis its safety analysis.
Materials and methods
Chemicals and media
2,4,6-Tripyridyl-S-triazine (TPTZ) reagent was procured from Sisco Research laboratories (SRL, Gurugram, India). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), amino acid kit, De Man, Rogosa and Sharpe (MRS) broth, potato dextrose agar (PDA), Muller–Hinton Agar (MHA), Luria broth (LB) were procured from Hi-media (Mumbai, India) and stored at 4 °C.
Bacterial strains
Bacterial strains Lacticaseibacillus rhamnosus GG MTCC #1408, Lactiplantibacillus plantarum MTCC #1407, Staphylococcus aureus MTCC #3160, Escherichia coli MTCC#585, Listeria monocytogenes MTCC#839, Proteus mirabilis MTCC#425, Klebsiella pneumoniae MTCC #109 and Saccharomyces cerevisiae MTCC #786 were procured from Microbial type Culture Collection, Institute of Microbial Technology (IMTECH), Chandigarh, India. Lactobacillus acidophilus NCDC #15 was procured from National Dairy Research Institute, Karnal, India. Four Lactic acid bacteria (LAB) (Pediococcus acidilactici BNS5B, Lactiplantibacillus pentosus GSSK2, Limosilactobacillus fermentum B3GS5, Limosilactobacillus fermentum PUM) isolated from different sources and well characterized for their potential probiotic attributes in our research laboratory were employed. All the probiotic strains and other bacterial strains were grown in MRS and LB, respectively, by incubating at 37 °C for 24 h and were sub-cultured regularly after an interval of 15 days. However, to maintain the phenotypic and genotypic characteristics, these bacterial cultures were preserved in 50% glycerol stored at − 20 °C while yeast strain was grown in glucose yeast extract broth (GYE; 0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 1% glucose) and maintained on PDA slants and stored at 4 °C.
Cell culture
HEK-293 and THP-1 cell lines were procured from National Center for Cell Science, Pune, India, and were maintained in RPMI-1640 supplemented with penicillin 10 U/mL, streptomycin 100 µg/mL, respectively, and 10% (v/v) fetal bovine serum, by incubating at 37 °C in 5% CO2 incubator.
Processing of Tinospora cordifolia (giloy) stem
Giloy stem was procured from local nursery of Chandigarh, India, washed properly, cut into small pieces, added to distilled water containing flask at a ratio of 10% (w/v), autoclaved (referred as stem aqueous extract) and were used for further experiments.
Inoculum preparation
Saccharomyces cerevisiae was grown in sterilized GYE broth at 30 °C for 24 h on a rotary shaker, cold centrifuged (4000g for 15 min), washed, suspended at a concentration of 108 cells/mL and referred to as pre-inoculum. The inoculum was prepared by transferring 10 ml of pre-inoculum to 100 ml of stem aqueous extract, the total soluble solid (TSS) of the mixture was adjusted at 5 °Brix (°Bx) with cane sugar followed by incubation at 30 °C on a rotary shaker for 24 h (Swami et al. 2016).
Fermentation of Tinospora cordifolia (giloy) stem
For wine production, 100 ml giloy stem aqueous extract was supplemented with cane sugar to adjust TSS to 20 °Bx, pH was adjusted to 4 with citric acid/sodium bicarbonate, followed by the addition of 100 ppm of potassium metabisulfite and was referred as production media. Thereafter, it was inoculated with 10% (v/v) inoculum of S. cerevisiae (108 CFU/ml) and incubated for fermentation at 30 ± 2 °C, was mixed 2–3 times a day and the progress in fermentation was monitored at a regular interval of 24 h by analyzing TSS, pH, ethanol, and total phenolic compounds (Chauhan et al. 2015).
Physio-chemical analysis of wine
Estimation of TSS and pH
TSS was measured in terms of °Bx, using ERMA hand refractometer having a range of 0–32 °Bx and pH was recorded using the pH meter (Max Electronics, India).
Ethanol estimation
Briefly, 1 mL wine sample was transferred to 250 mL round bottom distillation flask diluted with 30 mL distilled water and distilled at 80–90 °C. The distillate was collected in 50 mL volumetric flask containing 25 mL of potassium dichromate reagent and the distillate containing alcohol was collected, heated in water bath at 70 °C for 20 min, cooled and volume was made up to 50 mL with distilled water. Alcohol concentration was measured by measuring optical density at 600 nm (Caputi et al. 1968).
Fermentation efficiency was calculated as.
Ethanol obtainable from 1 g glucose, according to Gay-Lussac’s equation is 0.511 g which comes.
Estimation of total phenolic content
Briefly, to 1 mL of diluted wine sample, 1 mL of Folin–Ciocalteau reagent was added; after 3 min, 3 mL of 20% Na2CO3 was added, kept for 30 min with intermittent shaking and absorbance was measured at 765 nm. Results were expressed as µg/mL (Rathee et al. 2006).
Estimation of total antioxidant capacity
Briefly, to 3 ml working FRAP, 100 μL of wine sample was added, kept at room temperature for 10 min and absorbance was measured at 593 nm. Results were expressed as µmol/L (Benzie and Strain 1996).
Standardization of environmental factors and nutritional factor for production of herbal wine from giloy stem using one variable at a time (OVAT)
This was carried out by analyzing the effect of pH of the medium, incubation temperature, inoculum size, and sugar concentration in the medium by OVAT. Under each set of experiments, only the factor studied was varied and rest of the conditions was kept constants.
Effect of temperature: The effect of temperature was studied by incubating the inoculated production media at different temperatures such as 15 °C, 20 °C, 25 °C, 30 °C, 35 °C
Effect of pH: The effect of pH was studied by varying the pH (3.0–7.0) of the production media using citric acid or sodium bicarbonate
Effect of sugar concentration: The effect of sugar concentration was studied by adjusting the TSS in the production media at 10 °Bx, 15 °Bx, 20 °Bx, 25 °Bx, 30 °Bx
Effect of inoculum size: The effect of inoculum level was studied by inoculating the production media with varying levels of inoculums (2–12% v/v) having 108 viable cells/ml.
The effect of various nutritional factors was studied by supplementing the production medium, separately with different nitrogen source [diammonium hydrogen phosphate (1.88 g), ammonium dihydrogen phosphate (3.2 g), diammonium sulfate (1.88 g), ammonium chloride (1.52 g), peptone(2.76 g), urea (1.76 g), yeast extract (4 g)], mineral [K2HPO4 (3.5 g), MgSO4 (0.75 g), CaCl2 (9 mg), ZnSO4 (9 mg), FeSO4 (6 mg),MnCl2 (2 mg), CoCl2 (0.6 mg), CuSO4 (0.6 mg), KCl (0.2 mg), NaCl (0.2 mg)], amino acids [alanine (24 mg), arginine (62.5 mg), aspartic acid (7.5 mg), cysteine (2 mg), glutamine (84.5 mg), glutamic acid (20 mg), glycine (3 mg), histidine (5.5 mg), isoleucine (5.5 mg), leucine (8 mg), lysine(3 mg), methionine (5 mg), phenylalanine (6.5 mg), proline (10 mg), serine (13 mg), threonine (12.5 mg), tryptophan (30 mg), tyrosine (3 mg),valine (7.5 mg) and vitamins[biotin(3 mg), niacin(3 mg), thiamine(3 mg), inositol(15 mg), folic acid(3 mg), pyridoxine(3 mg), pantothenic(3 mg), riboflavin(3 mg)].
Statistical optimization of screened parameter by response surface methodology (RSM) using central composite design (CCD)
On the basis of OVAT, variables positively affecting the herbal wine (HW) production from giloy stem were selected. Further, RSM was employed to optimize the concentration of selected variable for wine production in an experimental plan of 53 trials keeping other factors constant. Regression analysis was performed on the data obtained from the design experiments. Experimental designs were generated and analyzed using the statistical software package Design-Expert 13.0 Stat-Ease Inc. Minneapolis MN USA. RSM with CCD was employed to optimize the parameters, viz. concentration of ethanol (X1), total phenolic content (X2), and total antioxidant capacity (X3). The response values were determined by the average of the two independent experiments. After the conduct of experiment, a second-order quadratic equation was fitted to evaluate the effect of each independent variable on the response and was used to construct 3D plots.
where Y is the measured response (ethanol, total phenolic content, and total antioxidant capacity), βo is the model constant, βi is coefficient of linear effect, βii is coefficient of quadratic effect, βij is coefficient of interaction effect, and ε is the error.
Data analysis
The resulting model was analyzed using one-way ‘analysis of variance’ (ANOVA) with p and F values also determined. Contour plots were also obtained using Design-Expert software (Version 13) to illustrate the relationship and interactive effects between the variables.
Validation of the experimental model
The production conditions for wine were optimized for maximum yield of ethanol, high antioxidant activity, and total phenol content based on the regression analysis. The responses were determined under the recommended conditions of the fermentation. To validate the model, predicted values were compared with the experimental value.
Preparation of probiotic-supplemented herbal wine (PSHW)
Seven probiotic cultures, i.e., L. rhamnosus GG, L. plantarum, L. acidophilus, P. acidilactici BNS5B, L. pentosus GSSK2, L. fermentum B3GS5, L. fermentum PUM were grown separately in MRS broth at 37 °C for 18 h, cold centrifuged at 4000g for 10 min, washed and suspended (1 × 109 CFU/ml) in prepared wine and kept at 4 °C. Viability of each probiotic culture in wine was monitored at regular interval of 4 days for a period of 5 weeks by spread plate method. Probiotic culture showing maximum viability was selected for the supplementation to herbal wine and was referred as probiotic-supplemented herbal wine (Acevedo-Martinez et al. 2018).
Cell viability assay
Cytotoxic potential of both HW and PSHW was monitored on HEK-293 and THP-1 cell lines (Sharma et al. 2020). Briefly, 200 µL cells (2 × 104cells/mL) were seeded in 96-well microtiter plates and incubated at 37 °C for 24 h followed by addition of HW and PSHW (10–100 µL) separately in wells, incubated at 37 °C for 24 h. After this, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated at 37 °C for 4 h. Thereafter, media was removed and blue formazan crystals formed were solubilized by the addition of 200 µL of dimethyl sulfoxide (DMSO). Absorbance was measured at 570 nm using ELISA reader (Tecan Infinite M200, Switzerland). The red wine was used as the control and processed in the same manner as HW and PSHW whereas untreated cells were taken as a positive control. Results were expressed in terms of percentage survival, where OD of untreated cell was taken as 100% live cell. All tests were performed in triplicate and repeated thrice.
Antibacterial efficacy of prepared wine
Antibacterial activity of prepared HW and PSHW against both Gram-positive (Staphylococcus aureus, Listeria monocytogenes) and Gram-negative (Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae) was performed by agar well diffusion assay (Swami et al. 2016). Briefly, MH agar plates were spread plated with 100 µl actively grown cultures (105 CFU/ml) followed by punching of wells (8 mm) in the agar plates aseptically. Four wells were punched in each MH agar plate and 100 µl of each HW and PSHW were added separately to the wells. Additionally, 100 µl of ethanol (10% v/v) and unfermented giloy juice were also added, separately and incubated at 37 °C for 24 h and monitored for zones of inhibition (mm).
LC-Q-ToF–MS metabolic profiling of wine
The MS spectrometry was carried out by LC–MS instrument (waters Micromass Q-ToF Micro) having quadrupole time of flight, mass equipped with electrospray ionization. 20 µl of the wine sample was separated on a C18 column (250 mm 4.6 mm 5 mm) using a mobile phase comprising methanol (A) − 0.1% formic acid (B) under gradient elution. The linear gradient increased from 10 to 17% A in 7 min, and increased to 30% A in another 1 min. Then, the solution A was continuously increased from 30 to 90% in 12 min. Finally, the solution A was linearly decreased back to the initial condition of 10%. Analysis was carried out in total chromatography ion mode with positive ESI interface. For MS, following parameters were used, desolyation gas: 550 L/h, cone gas: 35 L/h, desolvation temperature: 300 °C, source temperature: 110 °C, capillary voltage: 3000 V, cone voltage: 30 V. Identification was done on the basis of the molecular ion mass and compared with literature data.
Statistical analysis
A statistical analysis system, Design- Expert 13 Software was used for the statistical and graphical analysis. p value of less than 0.001 was considered statistically significant. All the results were expressed as mean ± standard deviation.
Results and discussion
Increasing health concern of people has prompted the interest toward the development of health promoting foods or physiologically active food components, herbal wines, are one such infusion that confers the health benefits on host beyond the basic nutrition. Additionally, the foods containing probiotic microorganism have gained much attention as probiotic show tremendous health benefits such as improve intestinal microbiota balance, anti-obesity, and anti-diabetic (Xavier-Santos et al. 2019; Khanna et al 2020). Production quality of wine depends on various factors such as fermentation temperature, pH, inoculum size, sugar content, and various nutritional elements. Therefore, the current research was headed toward understanding the nutritional factors, their interactions with each other, and physical factors vis-à-vis its safety assessment in vitro using HEK-293 and THP-1 cells.
Standardization of environmental and nutritional factors for production of herbal wine from giloy stem using OVAT
Effect of temperature on giloy stem fermentation
The optimum temperature for giloy stem fermentation was found to be 30 °C with an ethanol content of 7.3% (v/v), total phenolic content of 416.1 µg/ml, and fermentation efficiency of 76% whereas at 25 °C, ethanol content was 6.7% (v/v) with total phenolic content of 361.7 µg/ml and 61% fermentation efficiency. However, further increase in temperature (35 °C) resulted into decreased levels of ethanol (4.6% (v/v), total phenolic content (240.6 µg/ml), and fermentation efficiency (53%). Temperature is one of the most important physical parameters influencing the growth of the starter culture for fermentation and it was perceived that 30 °C is the optimum temperature for fermentation process and is in accordance with earlier studies (Singh et al. 1998; Reddy et al. 2005; Alagesan et al. 2016). These scientists have also reported the highest levels of ethanol and total phenolics at 30 °C in fruit wines made from amla, papaya, mango, kinnow. Although it is well known that every 10 °C rise in temperature doubles the chemical rate of reaction, increase in temperature also increases cell sensitivity to toxic effect of alcohol due to membrane fluidity leading to premature end of fermentation. Therefore, resulting into reduced fermentation efficiency at temperature above 30 °C whereas at low temperature (below 25 °C), fermentation rate is downregulated due to prolonged lag phase (Alagesan et al. 2016).
Effect of TSS on giloy stem fermentation
It was observed that ethanol production increased with TSS up to 20 °Bx resulting in maximum ethanol level of 7.5%, total phenolic content of 450.6 µg/ml, and fermentation efficiency of 79%. Surprisingly, further increase in TSS to 25˚Bx resulted in reduced production of ethanol, i.e., 6.5%, total phenolic content of 346.4 µg/ml and fermentation efficiency of 74% whereas at 10 °Bx, minimum ethanol content of 3.2% was achieved having total phenolic content of 263.6 µg/ml and fermentation efficiency of 68%. The reduced concentration of ethanol at 10 °Bx may be due to less availability of sugar to be fermented by the yeast cell. However, the reduced level of ethanol with increased TSS (beyond 25 °Bx) can be ascribed due to the development of stress causing disturbance in the osmotic gradient through the plasma membrane, eventually leading to decreased growth or loss of yeast cell viability (Fiscal et al. 2016; Satav et al. 2016; Jackson 2020).
Effect of inoculum size on giloy stem fermentation
A gradual increase in ethanol production was observed with increase in inoculum size and 10% (v/v) inoculum was found to be the best for the production of ethanol (7.6%) with total phenolic content of 453.3 µg/ml and fermentation efficiency of 77% from giloy herbal wine. However, further increase in inoculum size to 12% (v/v) resulted in decreased ethanol (5.4%), total phenol content (323.8 µg/ml), and fermentation efficiency (52%), respectively. Scientists have also observed that 10% inoculum was best for kinnow, pear, jamun, palm, china rose and litchi wine production (Singh et al 1998; Swami et al 2016). The reduced level of ethanol with increased inoculum size may be due to toxic metabolites leading to nutritional deficit in the fermentation media, thus leading to alteration in growth condition (Miljic et al. 2014; Tiwari et al. 2017).
Effect of pH on giloy stem fermentation
Highest ethanol level of 8.3%, total phenolic content of 473.7 µg/ml, and 76% fermentation efficiency were obtained at pH 4.5 followed by 7.6% ethanol, 426.3 µg/ml total phenolic content, and 66%fermentation efficiency at pH 4 whereas at pH 3, minimum ethanol content of 5.2%, total phenolic content of 256.7 µg/ml and fermentation efficiency of 62% were obtained. The optimum pH for the herbal wine production was 4.5 and is in agreement with the earlier reports (Swami et al. 2016; Kavitha and Kannahi 2018). These scientists have also found that pH 4.5 was optimum for the production of wine from different sources, e.g., vegetable, jamun, carambola, kinnow. pH control during fermentation is important, because yeast growth is supported at mild acidic pH while growth of harmful bacteria is inhibited. However, alkaline pH results into acetic acid production rather than alcohol due to increased activity of aldehyde dehydrogenase (Mathewson 1980).
Screening of nutritional parameters by OVAT
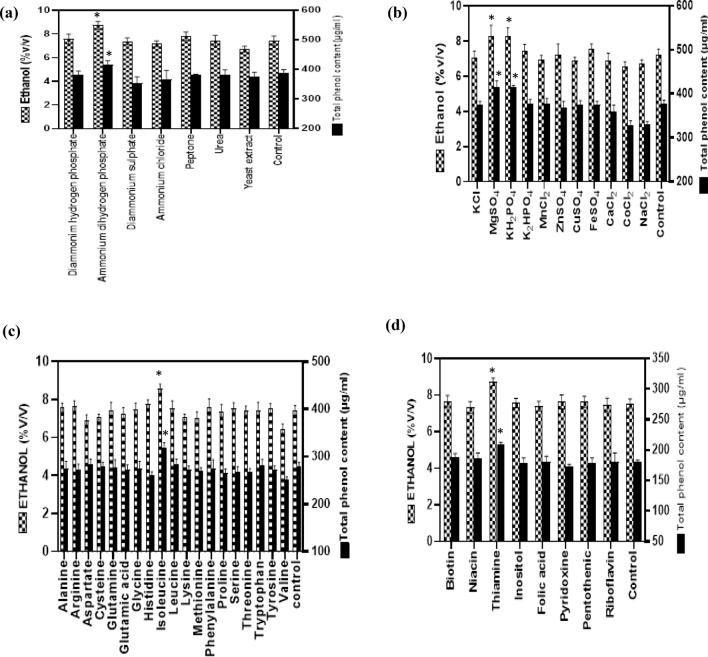
Yeast encounters a variety of nitrogen sources in its natural habitat but not all the nitrogen sources promote yeast growth. Among seven different nitrogen sources tested at equivalent nitrogen level, (NH4) H2PO4 was most effective in enhancing the production of alcohol (8.7% (v/v) with total phenolic content of 540 µg/ml (Fig. 1a). Nitrogen deficiency is known for the stuck and sluggish fermentation during wine production, and to combat this, ammonium salts have been found to show positive effect on fermentation process. (NH4) H2PO4 produced the maximum alcohol and total phenol content; and is in collaboration with earlier studies where ammonium salts had positive effect on fermentation process (Pretorius 2000; Cruz et al. 2003).
Fig. 1.
Effect of supplementation of: a nitrogen source; b mineral salts; c amino acids; d vitamins on giloy stem fermentation. Results are expressed as mean ± standard deviation, *p < 0.05 versus control
Beside nitrogen source, fermentation is also affected by minerals deficiency. Therefore, from the various mineral sources employed, KH2PO4 and MgSO4 showed highest alcohol content of 8.1% (v/v), 8.3% (v/v) with total phenolic content of 542 µg/ml and 539 µg/ml, respectively (Fig. 1b). Mineral deficiency also affects the yeast cell homeostasis, enzymatic activity as well as intracellular pH. Thus, the addition of KH2PO4 and MgSO4 in medium led to maximize both alcohol as well as total phenol content and is in agreement with earlier studies (Slininger et al. 2006; Soni et al. 2009; Schwarz et al. 2020). These scientists have also reported that potassium and magnesium are major cations required for maintaining the desired ionic environment that facilitates the growth of yeast cell.
Of the various amino acids and vitamins, isoleucine and thiamine supplementation resulted in the highest ethanol level of 8.5%(v/v) and 8.4% (v/v), with total phenolic content of 546 µg/ml and 526 µg/ml, respectively (Fig. 1c, d). Out of all the amino acids, isoleucine had maximum alcohol and total phenol content and is in accordance with Slininger et al. (2006). These scientists have also demonstrated that amino acids have positive impact on ethanol production as they act as a catalyst for the supply of nitrogen and building of protein during fermentation; beside this, amino acids also protect yeast cells against various stress factors, which would reduce the alcohol production. Labuschagne and Divol (2021) have observed that thiamine plays an important role in protection of yeast against external stress factors as well as serves as a growth factor.
Optimization of screened variant using response surface methodology
RSM using Central Composite Design was employed to determine the optimum nutrients concentration that yield high ethanol level, total phenol content and antioxidant activity and understand the interaction between 5 selected variables, i.e., (NH4) H2PO4, KH2PO4, MgSO4, isoleucine, thiamine in an experiment of 53 runs. Concentration of ethanol obtained varied from 4.7 to 10% (v/v), total phenolic content from 364.1 to 791.5 µg/ml, and total antioxidant capacity from 56.1 to 160.4 µg/ml in an experiment of 53 runs whereas maximum yield of ethanol, total phenolic content, and antioxidant capacity were obtained at a run 6. The fermentation parameters were optimized based on this information for maximum ethanol production and for the better antioxidant and phenolic extraction in the wine.
Evaluation for the validation of fitted model was carried out and the significance of all terms in polynomial was conducted by F test (ANOVA). The results of analysis of variance (ANOVA) revealed that the model was significant (Tables 1, 2, 3). The actual and predicted values were found to be reasonably close to each other, indicating that the model has progressed satisfactorily. The model revealed the 95% confidence level to be statistically significant, with an F value of 77.78, 485.18, and 112.61 for alcohol, phenolic, and antioxidant content, respectively. The fitness of model was verified by R2 and adjusted R2 values for all the three responses. The R2 and adjusted R2 values are in reasonable agreement and showed that the model has established the relationship between the chosen parameters. Moreover, the model was found to be significant for the responses as the lack of fit term was non-significant and p values < 0.0001 indicated that the linear, interactive, and squared terms significantly influenced the responses. The coefficient of determination (R2) values of 0.9382, 0.9906, 0.9629 for ethanol, total phenolic content, and antioxidant capacity indicated that the model provides accurate description of the experimental data (Table 4). All this data confirmed that the model can be used for maximum alcohol, phenol, and antioxidant production. Similarly, various studies have observed the influence of nitrogen, minerals, amino acids, and vitamins in wine production and it was confirmed that these factors play key role in the wine production as well as influencing the quality of wine (Joshi and Kumar 2015; Schwarz et al. 2020).
Table 1.
Analysis of variance (ANOVA) for Response Surface Quadratic Model for alcohol
| Source | Sum of square | df | Mean square | F value |
p value prob > F |
|
|---|---|---|---|---|---|---|
| Block | 86.85 | 2 | 43.42 | |||
| Model | 30.23 | 20 | 1.51 | 351.24 | < 0.0001 | Significant |
| A-NH4H2PO4 | 0.40 | 1 | 0.40 | 93.43 | < 0.0001 | |
| B-KH2PO4 | 0.013 | 1 | 0.013 | 2.93 | 0.0973 | |
| C-MgSO4 | 1.70 | 1 | 1.70 | 395.45 | < 0.0001 | |
| D-Isoleucine | 6.65 | 1 | 6.65 | 1545.57 | < 0.0001 | |
| E-Thiamine | 3.89 | 1 | 3.89 | 903.57 | < 0.0001 | |
| AB | 0.58 | 1 | 0.58 | 134.91 | < 0.0001 | |
| AC | 0.33 | 1 | 0.33 | 76.71 | < 0.0001 | |
| AD | 0.27 | 1 | 0.27 | 62.35 | < 0.0001 | |
| AE | 0.26 | 1 | 0.26 | 61.50 | < 0.0001 | |
| BC | 0.016 | 1 | 0.016 | 3.66 | 0.0653 | |
| BD | 0.033 | 1 | 0.033 | 7.70 | 0.0094 | |
| BE | 0.78 | 1 | 0.78 | 180.84 | < 0.0001 | |
| CD | 1.08 | 1 | 1.08 | 251.95 | < 0.0001 | |
| CE | 0.94 | 1 | 0.94 | 217.30 | < 0.0001 | |
| DE | 3.91 | 1 | 3.91 | 909.39 | < 0.0001 | |
| A2 | 0.25 | 1 | 0.25 | 57.32 | < 0.0001 | |
| B2 | 1.12 | 1 | 1.12 | 260.54 | < 0.0001 | |
| C2 | 1.38 | 1 | 1.38 | 320.38 | < 0.0001 | |
| D2 | 2.84 | 1 | 2.84 | 660.52 | < 0.0001 | |
| E2 | 2.49 | 1 | 2.49 | 578.52 | < 0.0001 | |
| Residual | 0.13 | 30 | 4.303E−003 | |||
| Lack of fit | 0.027 | 22 | 1.212E−003 | 0.095 | 1.0000 | Not significant |
| Pure error | 0.10 | 8 | 0.013 | |||
| Cor total | 117.20 | 52 |
Table 2.
Analysis of variance (ANOVA) for Response Surface Quadratic Model for total phenol content
| Source | Sum of square | df | Mean square | F value |
p value prob > F |
|
|---|---|---|---|---|---|---|
| Block | 2.370E+005 | 2 | 1.185E+005 | |||
| Model | 2.194E+005 | 20 | 10,968.80 | 485.18 | < 0.0001 | significant |
| A-NH4H2PO4 | 53.13 | 1 | 53.13 | 2.35 | 0.1358 | |
| B-KH2PO4 | 1291.63 | 1 | 1291.63 | 57.13 | 0.0973 | |
| C-MgSO4 | 6840.84 | 1 | 6840.84 | 302.59 | < 0.0001 | |
| D-Isoleucine | 40,011.95 | 1 | 40,011.95 | 1769.85 | < 0.0001 | |
| E-Thiamine | 50,901.09 | 1 | 50,901.09 | 2251.51 | < 0.0001 | |
| AB | 76.87 | 1 | 76.87 | 3.39 | 0.0756 | |
| AC | 31.80 | 1 | 31.80 | 1.41 | 0.2449 | |
| AD | 342.57 | 1 | 342.57 | 15.15 | 0.0005 | |
| AE | 38.50 | 1 | 38.50 | 1.70 | 0.2018 | |
| BC | 18.45 | 1 | 18.45 | 0.82 | 0.3735 | |
| BD | 447.75 | 1 | 447.75 | 19.81 | 0.0001 | |
| BE | 29.84 | 1 | 29.84 | 1.32 | 0.2597 | |
| CD | 2062.43 | 1 | 2062.43 | 91.23 | < 0.0001 | |
| CE | 929.88 | 1 | 929.88 | 41.13 | < 0.0001 | |
| DE | 1538.74 | 1 | 1538.74 | 68.06 | < 0.0001 | |
| A2 | 26,855.57 | 1 | 26,855.57 | 1187.91 | < 0.0001 | |
| B2 | 34,624.20 | 1 | 34,624.20 | 1531.54 | < 0.0001 | |
| C2 | 15,560 | 1 | 15,560 | 688.27 | < 0.0001 | |
| D2 | 22,425.06 | 1 | 22,425.06 | 991.93 | < 0.0001 | |
| E2 | 482.29 | 1 | 482.29 | 21.33 | < 0.0001 | |
| Residual | 678.23 | 30 | 22.61 | |||
| Lack of fit | 545.14 | 22 | 24.78 | 1.49 | 0.2889 | Not significant |
| Pure Error | 133.09 | 8 | 16.64 | |||
| Cor total | 4.571E+005 | 52 |
Table 3.
Analysis of variance (ANOVA) for Response Surface Quadratic Model for antioxidant capacity
| Source | Sum of square | df | Mean square | F value |
p value prob > F |
|
|---|---|---|---|---|---|---|
| Block | 10,449.99 | 2 | 5225.00 | |||
| Model | 14,558.03 | 20 | 727.90 | 112.61 | < 0.0001 | Significant |
| A-NH4H2PO4 | 22.35 | 1 | 22.35 | 3.46 | 0.0728 | |
| B-KH2PO4 | 77.01 | 1 | 77.01 | 11.91 | 0.0017 | |
| C-MgSO4 | 523.45 | 1 | 523.45 | 80.98 | < 0.0001 | |
| D-Isoleucine | 1807.85 | 1 | 1807.85 | 279.65 | < 0.0001 | |
| E-Thiamine | 3839.64 | 1 | 3839.64 | 594.00 | < 0.0001 | |
| AB | 192.57 | 1 | 192.57 | 29.79 | < 0.0001 | |
| AC | 28.69 | 1 | 28.69 | 4.44 | 0.0436 | |
| AD | 1158.01 | 1 | 1158.01 | 179.15 | < 0.0001 | |
| AE | 32.20 | 1 | 32.20 | 4.98 | < 0.0001 | |
| BC | 192.57 | 1 | 192.57 | 29.79 | < 0.0001 | |
| BD | 573.76 | 1 | 573.76 | 88.76 | 0.0236 | |
| BE | 36.77 | 1 | 36.77 | 5.69 | < 0.0001 | |
| CD | 211.67 | 1 | 211.67 | 32.75 | < 0.0001 | |
| CE | 2330.74 | 1 | 2330.74 | 360.57 | < 0.0001 | |
| DE | 2597.40 | 1 | 2597.40 | 401.82 | < 0.0001 | |
| A2 | 273.51 | 1 | 273.51 | 42.31 | 0.0033 | |
| B2 | 65.70 | 1 | 65.70 | 10.16 | 0.0580 | |
| C2 | 25.12 | 1 | 25.12 | 3.89 | < 0.0001 | |
| D2 | 167.30 | 1 | 167.30 | 25.88 | < 0.0001 | |
| E2 | 320.69 | 1 | 320.69 | 49.61 | < 0.0001 | |
| Residual | 193.92 | 30 | 6.46 | |||
| Lack of fit | 111.91 | 22 | 5.09 | 0.50 | 0.9076 | Not significant |
| Pure error | 82.01 | 8 | 10.25 | |||
| Cor total | 25,201.94 | 52 |
Table 4.
The fitted quadratic model in terms of coded variables for Y1, Y2, and Y3 responses
| Response | 2nd order polynomial equation | p value | F value | R2 | R2 adjusted |
|---|---|---|---|---|---|
| Ethanol | Y1 = + 6.02 − 0.11A + 0.014B − 0.21C − 0.40D − 0.31E − 0.12AB + 0.10AC + 0.073AD + 0.073AE − 0.020BC − 0.052 + 0.14BE + 0.17CD + 0.16CE + 0.37DE + 0.074A2 + 0.17B2 + 0.20C2 + 0.28D2 + 0.26E2 | 0.0001 | 77.8 | 0.9383 | 0.9685 |
| Total phenol content | Y2 = + 512.58 + 1.15A − 5.68B − 13.08C − 31.63D − 36.67E − 1.55AB − 1.00AC + 3.27AD + 1.10AE + 0.76BC + 3.74BD + 0.97BE + 8.03CD + 5.39CE + 6.93DE + 28.42A2 + 32.27B2 + 21.63C2 + 25.97D2 + 3.81E2 | 0.0001 | 485.18 | 0.9906 | 0.9949 |
| Total antioxidant capacity | Y3 = + 85.63 + 0.75A − 1.39B − 3.62C − 0.672D − 9.80E + 2.45AB − 0.95AC + 6.02AD + 1.00AE − 2.45BC + 4.23BD + 1.07BE + 2.57CD + 8.53CE + 9.01DE + 2.87A2 + 1.41B2 − 0.87C2 + 2.24D2 + 3.11E2 | 0.0001 | 112.61 | 0.9626 | 0.9781 |
Survivability of different probiotic strain in prepared wine
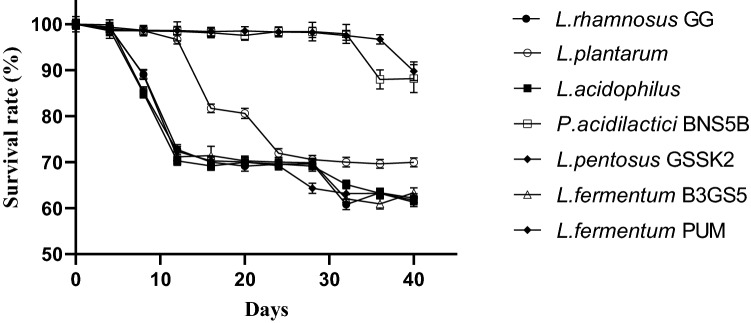
After supplementation of prepared herbal wine with seven different probiotic strains separately, survival of the probiotic cells was monitored for 5 consecutive weeks at an interval of 4 days. It was perceived that out of seven probiotics supplemented strains, L. pentosus GSSK2 exhibited highest survival rate of 88.6% for a period of 4 weeks followed by P. acidilactic BNS5B (85.2%) and L. rhamnosus GG (58%), respectively (Fig. 2). In the present study, L. pentosus GSSK2 showed maximum survivability at 4 °C for a period of 4 weeks, thereafter cell count decreased to 106 CFU/ml, may be due to stringent conditions in the wine. The storage temperature also influences the viability of probiotic bacteria in herbal, fruit, and dairy matrices; due to this, most of the beverages supplemented with probiotics are stored at 4 °C, as low temperature attenuates the acidification process and enhances the survival of probiotic organism (Khatoon and Gupta 2015; Acevedo-Martinez et al. 2018).
Fig. 2.
Survival rate (%) of different probiotic strains in the prepared giloy stem wine. Results are expressed as mean ± standard deviation
Cytotoxicity assay
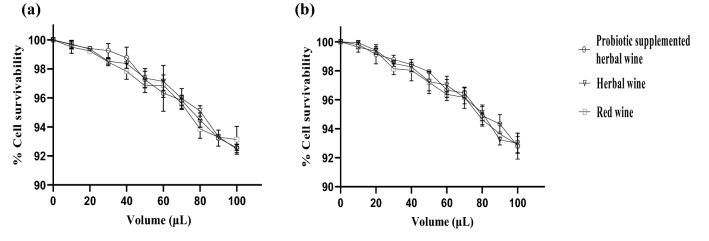
The cytotoxicity potential of HW, PSHW, and red wine on HEK-293 and THP-1 cell lines was assessed and it was interesting to observe that cell lines (HEK-293 and THP-1) treated either with HW, PSHW or red wine at different volumes (10–80 µl) showed no cytotoxic effect whereas 100 µl had mild effect on the viability of cells, as cells treated with either HW or PSHW had 92% viability (THP-1 and HEK-293) whereas red wine had cell viability of 92 and 93% on HEK-293 and THP-1 cell lines, respectively (Fig. 3a, b). Cytotoxicity is one of the most important parameter to monitor the safety of any agent in vitro, as the viable cells are good indicators for safety of the compound. It was interesting to observe that treatment either with HW, PSHW or red wine was safe for 24 h as 100% viability was observed upto 80 µl whereas 100 µl of HW, PSHW, and red wine showed very mild toxicity (7–8% dead cell) and is in concordance with earlier studies (Pozo-Guisado et al. 2002; Goktas et al. 2017). These scientists have also found that red wine was safe and has no cytotoxic effects on various cell lines, i.e., V79, MDA MB 231 even after 24 h incubation.
Fig. 3.
Percent cell survivability (THP-1 and HEK293) at different volume of herbal wine, probiotic-supplemented herbal wine, and red wine after 24 h of incubation at 37 °C: a THP-1; b HEK293. Results are expressed as mean ± standard deviation
Antibacterial activity of wine
It was interesting to observe that both HW and PSHW exhibited antibacterial potential against both Gram-positive (S. aureus, L. monocytogenes) and Gram-negative (E. coli, P. mirabilis, K. pneumoniae). Interestingly, PSHW showed better antibacterial activity than HW while giloy juice and 10% ethanol did not exhibit any antibacterial activity (Table 5). Zone of inhibition against both Gram-positive and Gram-negative bacteria confers the antibacterial activity of prepared HW and PSHW which may be attributed mainly to polyphenolic content of wine (Vaquero et al. 2007). Moreover, larger inhibition zone of PSHW may be ascribed to various bioactive metabolites produced by probiotics such as short chain fatty acid, bacteriocin, exopolysaccharides, known to have antibacterial potential (Friedman 2015; Durva et al. 2020).
Table 5.
Antimicrobial activity of herbal wine and probiotic-supplemented herbal wine
| Bacterial culture | Zone of inhibition (mm) | |
|---|---|---|
| Herbal wine | Probiotic-supplemented herbal wine | |
| K. pneumoniae | 19 ± 1.24 | 21 ± 1.89 |
| S. aureus | 19 ± 0.35 | 20 ± 1.21 |
| P. mirabilis | 18 ± 1.54 | 20 ± 2.12 |
| E. coli | 18 ± 1.27 | 19 ± 0.87 |
| L. monocytogenes | 19 ± 0.32 | 21 ± 1.03 |
Results are mean ± standard deviation from three independent experiments
LC-Q-ToF–MS metabolic profiling of wine
LC–MS/MS with TOF-ESI and positive ionization mode was performed for qualitative estimation of the chemical nature of phenolic compounds in wine because of its high selectivity and sensitivity. Gradient RP-HPLC with absorbance detection and MS with an electron interface were used for the analysis. Five structurally distinct groups were identified in probiotic supplemented herbal wine, i.e., alkaloids (berberine, choline, tembetarine, magnoflorine, tinosporin, palmitine), diterpenoid lactone (tinosporide, columbin), glycosides (syringin, furanoid diterpene), steroids (ecdysterone, makisterone, β-sitosterol, 20β-hydroxyecdysone), aliphatic (heptacosanol, nonacosan-15-one) and other compounds beside these. The presence of phytochemicals like jatrorrhizine, palmatine, furanoid diterpene, etc., advocates the medicinal efficacy of the probiotic-supplemented herbal wine (Table 6). More specifically, LC–MS data have revealed the presence of various bio-active compounds attributing to the antibacterial, anti-oxidant, and anti-inflammatory potential of the probiotic-supplemented herbal wine and is in correlation with earlier studies (Swami et al. 2016; Tiwari et al. 2017).
Table 6.
Bioactive compounds identified in probiotic supplemented herbal wine using LC-Q-ToF-MS
| Compounds | m/z | Biological activity |
|---|---|---|
| Tinosporide | 374.38 | Vasorelaxant, anti-hypertensive, anti-viral (Sriramaneni et al. 2010; Yang et al. 2010) |
| Furanoid diterpene | 390.2 | Inhibit NF-kappaB and act as nitric oxide scavenger to show anticancer activity, immunomodulation (Karpova et al.1991; Chen et al. 2001) |
| Ecdysterone | 480.6 | Anti-inflammatory, inhibits-TNF-α (Sundarraj et al. 2012) |
| Makisterone | 478.2 | Inhibits IL-6, IL-1β, anti-depressant (Wang et al. 2010), |
| Berberine | 336.9 | Antiviral, anticancer, antidiabetic (Upadhaya et al 2010; Rout et al. 2006) |
| Choline | 104.5 | Anticancer, anti-inflammatory, antidiabetic, hepatoprotective (Gupta et al. 2011; Patel et al. 2011) |
| Tembetarine | 344.2 | Neurological, immunomodulatory (Rout et al. 2006; Jagetia et al. 2006) |
| Magnoflorine | 342.9 | Anticancer, antidiabetic, anti-inflammatory (Upadhaya et al. 2010; Patel et al. 2011) |
| Dehydrodiscretamine | 324.2 | Neurological disorders, anticancer, hepatoprotective |
| Bergenin | 327.1 | Anti-oxidative, immunomodulatory (Gupta et al. 2011; Patel et al. 2006) |
| Gallic acid | 169.1 | Radical scavenger, anticancer, antidiabetic, anti-inflammatory (Rout et al. 2006) |
| Tinosporin | 406.9 | Neurological conditions, anti-diabetic, psychiatric condition (Jagetia et al. 2006) |
| Columbin | 358.4 | Induces apoptosis in leukemia by activating caspase-3 and bax, inhibits bcl-2, antiviral, anti-inflammatory (Kohno et al. 2002; Dhanasekaran et al. 2009) |
| Heptacosanol | 396 | Anticancer, inhibit TNF-α (Thippeswamy et al. 2008; Wang et al. 2010) |
| Nonacosan-15-one | 422.7 | Anti-inflammatory, inhibits IL-6 (De-Oliveria et al. 2012; Wang et al. 2010) |
| Syringin | 395.5 | Treat neurological conditions, immunomodulation (Yang et al. 2010) |
| Β-Sitosterol | 414.5 | Inhibits COX-2, TNF-α, IL-1β, IL-6 (Sundarraj et al. 2012) |
| 20β-Hydroxyecdysone | 481.8 | Inhibits TNF-α, IL-1β, IL-6 (Sundarraj et al. 2012) |
| Reticuline | 330.9 | Help in platelets aggregation, anti-inflammatory (Bala et al. 2015) |
| Menisperine | 356.4 | Anticancer, hepatoprotective, inhibit IL-6 (Bala et al. 2015) |
| Palmitine | 352.1 | Anticancer, anti-inflammatory (Sriramaneni et al. 2010; Yang et al. 2010) |
Based on the observation of present study, it can be asserted that optimization of fermentation process led to significant improvement in the bio-chemical characteristics of PSHW in terms of high phenolic and antioxidant capacity. Major phenolics and other bioactive compounds identified by LC–MS analysis depicted the presence of numerous health beneficial phytochemicals; thus, PSHW can be an effective antioxidant beverage against the disease mediated by oxidative stress.
Conclusion
Taken together, it can be stated that giloy can be used as the substrate for the production of probiotic-supplemented herbal wine having antibacterial, antioxidant, and anti-inflammatory potential. A TSS of 20 °Bx, temperature 30 °C, pH 4.5, inoculum size 10% (v/v), and nutritional factors such as ammonium dihydrogen phosphate, Mg2SO4, KH2PO4, isoleucine, and thiamine resulted into maximum alcohol, phenol, and antioxidant production. Interestingly, supplementation of herbal wine with probiotic L. pentosus GSSK2 was safe and had antibacterial and antioxidant potential, indicating its medicinal functionality. The novelty of such probiotic-supplemented herbal wine from giloy is that its anti-inflammatory and antioxidant potential can be used in the management of various lifestyle disease such as obesity, diabetes, hypertension, colorectal cancer, etc., that needs to be correlated both experimentally and clinically. However, an experimental study to validate the ameliorating potential of PSHW in metabolic syndrome is under progress.
Acknowledgements
Financial assistance provided by Council of Scientific and Industrial Research (CSIR); New Delhi is highly acknowledged. Authors are thankful to the Sophisticated Analytical Instrumentation Facility (SAIF), Chandigarh, Panjab University, India for LC–MS analysis
Author contribution
SK performed the experiment and wrote the manuscript; GS conceptualized the research and edited the manuscript; SKS analyzed the data and edited the manuscript.
Data availability
The presented data is available from the corresponding author on reasonable request.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Acevedo-Martínez E, Gutierrez-Cortes C, García-Mahecha M, Díaz-Moreno C. Evaluation of viability of probiotic bacteria in mango (Mangifera indica L. Cv., “Tommy Atkins”)beverage. DYNA (colombia) 2018;85(207):84–92. doi: 10.15446/dyna.v85n207.70578. [DOI] [Google Scholar]
- Alagesan CM, Panneerselvam A. Production, optimization and characterization of wine from papaya using Saccharomyces cerevisiae. Int J Curr Microbiol App Sci. 2016;3(3):1–7. [Google Scholar]
- Bala M, Pratap K, Verma PK, Singh B, Padwad Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J Ethnopharmacol. 2015;175(4):131–137. doi: 10.1016/j.jep.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as measurement of—antioxidant power: the Frap assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhise P, Morya S. The health sustainability of herbal wine bioactives towards different chronic diseases. J Pharma Innov. 2021;10(5):512–517. doi: 10.22271/tpi.2021.v10.i5g.6258. [DOI] [Google Scholar]
- Caputi A, Ueda M, Brown T. Spectrophotometric determination of ethanol in wine. Am J Enol Vitic. 1968;19:160–165. doi: 10.5344/ajev.1968.19.3.160. [DOI] [Google Scholar]
- Chauhan A, Swami U, Negi B, Soni SK. A valorized wine from aloe vera and Mentha arvensis and its LC-Q-ToF-MS metabolic profiling. Int J Food Ferment Technol. 2015;5(2):183–190. doi: 10.5958/2277-9396.2016.00013.1. [DOI] [Google Scholar]
- Chen S, Wu K, Knox R. Structure-function studies of DT-diaphorase (NQO1) and NRH: quinone oxidoreductase (NQO2) Free Radic Biol Med. 2001;29:276–284. doi: 10.1016/S0891-5849(00)00308-7. [DOI] [PubMed] [Google Scholar]
- Corbo MR, Bevilacqua A, Petruzzi L, Casanova FP, Sinigaglia M. Functional beverages: the emerging side of functional foods: commercial trends, research, and health implications. Compr Rev Food Sci Food Saf. 2014;13(6):1192–1206. doi: 10.1111/1541-4337.12109. [DOI] [Google Scholar]
- Cruz SH, Batistote M, Ernandes JR. Effect of sugar catabolite repression in correlation with the structural complexity of the nitrogen source on yeast growth and fermentation. J Inst Brew. 2003;109:349–355. doi: 10.1002/j.2050-0416.2003.tb00609.x. [DOI] [Google Scholar]
- De-Oliveria AM, Conserva LM, De-Souza Ferro JN, De-Almeida Brito F, LyraLemos RP, Barreto E. Antinociceptive and anti-inflammatory effects of octacosanol from the leaves of sabiceagrisea var. Grisea in mice. Int J Mol Sci. 2012;13:1598–1611. doi: 10.3390/ijms13021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran M, Baskar AA, Ignacimuthu S, Agastian P, Duraipandiyan V. Chemopreventive potential of epoxyclerodane diterpene from Tinospora cordifolia against diethyl nitrosamine induced hepyocellular carcinoma. Invest New Drugs. 2009;27:347–355. doi: 10.1007/s10637-008-9181-9. [DOI] [PubMed] [Google Scholar]
- Durva P, Aakifa A, Apoorva P, Anila G, Neil F, Pooja P, Miriam S, Pampi C. Antioxidant and Antibacterial properties of wine prepared from bananas. Res J Biotechnol. 2020;15(6):88–97. [Google Scholar]
- FAO/WHO . Report of a Joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. Rome: FAO/WHO; 2002. [Google Scholar]
- Fiscal RR, Chavez ACC. Development and physicochemical evaluation of wine from taro corms (Colocasia esculenta) Int J Sci Res. 2016;5(9):146–151. doi: 10.21275/ART20161422. [DOI] [Google Scholar]
- Food and Agriculture Organization (FAO) Functional foods: safety and regulation aspects. Rome: FAO; 2004. [Google Scholar]
- Friedman M. Antibiotic-resistant bacteria: prevalence in food and inactivation by food-compatible compounds and plant extracts. J Agric Food Chem. 2015;63(15):3805–3822. doi: 10.1021/acs.jafc.5b00778. [DOI] [PubMed] [Google Scholar]
- Goktas HG, Bacanli M, Kutluk B, Başaran AA, Başaran N (2017) Cytotoxic activity of resveratrol in different cell lines evaluated by MTT and NRU assays. https://jag.journalagent.com/tjps/pdfs/TJPS-40085-RESEARCH_ARTICLE-GOKTAS.pdf
- Gupta R, Sharma V. Ameliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by aflatoxin-b (1) in mice kidney. Toxicol Int. 2011;18:94–98. doi: 10.4103/0971-6580.84259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RS. Wine science: principles and applications (food science and technology) 5. USA: Academic press; 2020. [Google Scholar]
- Jagetia GC, Rao SK. Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biol Pharm Bull. 2006;29:460–466. doi: 10.1248/bpb.29.460. [DOI] [PubMed] [Google Scholar]
- Joshi VK, Kumar N. Optimization of low alcoholic bitter gourd apple beverage by applying Response surface methodology (RSM) Int J Food Ferment Technol. 2015;5(2):191–199. doi: 10.5958/2277-9396.2016.00008.8. [DOI] [Google Scholar]
- Karpova EA, Voznyi YV, Dudukina TV, Tsvetkva IV. 4-Trifluoromethylumbelliferyl glycosides as new substrates form revealing diseases connected with hereditary deficiency of lysosome glycosidases. Biochem Int. 1991;24:1135–1144. [PubMed] [Google Scholar]
- Kavitha P, Kannahi M. Fermentative production and optimization of wine from different vegetables. Int Bio Res. 2018;3(2):105–108. [Google Scholar]
- Khanna S, Walia S, Kondepudi KK, Shukla G. Administration of indigenous probiotics modulate high-fat diet-induced metabolic syndrome in Sprague Dawley rats. Antonie Van Leeuwenhoek. 2020;113:1345–1359. doi: 10.1007/s10482-020-01445-y. [DOI] [PubMed] [Google Scholar]
- Khatoon N, Gupta RK. Probiotics beverages of sweet lime and sugarcane juices and its physiochemical, microbiological & shelf-life studies. J Pharmacogn Phytochem. 2015;4(3):25–34. [Google Scholar]
- Kohno H, Maeda M, Tanino M, Tsukio Y, Ueda N, Wada K, et al. A bitter diterpenoid furano lactone columbine from calumbae Radix inhibits azoxy methane-induced rat colon carcinogenesis. Cancer Lett. 2002;183:131–139. doi: 10.1016/S0304-3835(02)00159-3. [DOI] [PubMed] [Google Scholar]
- Labuschagne P, Divol B. Thiamine: a key nutrient for yeasts during wine alcoholic fermentation. Appl Microbiol Biotechnol. 2021;105(3):953–973. doi: 10.1007/s00253-020-11080-2. [DOI] [PubMed] [Google Scholar]
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Velez E, Perdigon G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2017;74(2):115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- Mallappa RH, Rokana N, Duary RK, Panwar H, Batish VK, Grover S. Management of metabolic syndrome through probiotic and prebiotic interventions. Indian J Endocrinol Metab. 2012;16:20–27. doi: 10.4103/2230-8210.91178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson WS (1980) The Manual for the home and farm production of alcohol fuel. In: Farm production of alcohol fuel. J.A. Diaz publication.
- Miljic UD, Puskas VS. Influence of fermentation conditions on production of plum (Prunus domestica L.) wine: a response surface methodology approach. Hem Ind. 2014;68:199–206. doi: 10.2298/HEMIND130307044M. [DOI] [Google Scholar]
- Mirmiran P, Bahadoran Z, Azizi F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: a review. World J Diabetes. 2014;5(3):267–281. doi: 10.4239/wjd.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka T. Development of functional foods. Biosci Microb Food Health. 2014;33:117–128. doi: 10.12938/bmfh.33.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi B, Kumari SK, Shrestha J, Shrestha P, Basnet A, Tiwari I, Prasad AS. Morphology, biological activity, chemical composition, and medicinal value of Tinospora Cordifolia (willd.) Miers. Adv J Chem. 2021;3(1):36–54. doi: 10.22034/ajcb.2020.243751.1058. [DOI] [Google Scholar]
- Ozer B, Kirmaci HY. Functional milks and dairy beverages. Int J Dairy Technol. 2010;63:1–15. doi: 10.1111/j.1471-0307.2009.00547.x. [DOI] [Google Scholar]
- Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine. 2011;18:1045–1052. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 2002;64(9):1375–1378. doi: 10.1016/S0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- Pretorius IS. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast. 2000;16(8):675–729. doi: 10.1002/1097-0061(20000615)16:8<675::AID-YEA585>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Rathee JS, Hassarajani SA, Chattopadhyay S. Antioxidant activity of Mammea longifolia bud extracts. Food Chem. 2006;99(3):436–443. doi: 10.1016/j.foodchem.2005.08.020. [DOI] [Google Scholar]
- Reddy LVA, Reddy OVS. Production and characterization of wine from mango fruit (Mangifera indica L) World J Microbiol Biotechnol. 2005;21(8–9):1345–1350. doi: 10.1007/s11274-005-4416-9. [DOI] [Google Scholar]
- Rout GR. Identification of Tinospora cordifolia (Willd.) Miers ex Hook F & Thoms using RAPD markers. Z Naturforsch. 2006;61c:118–122. doi: 10.1515/znc-2006-1-221. [DOI] [PubMed] [Google Scholar]
- Satav PD, Pethe AS. Effect of pH on physicochemical parameters of wine produced from banana. Int J Curr Microbiol Appl Sci. 2016;5(2):608–614. doi: 10.20546/ijcmas.2016.502.068. [DOI] [Google Scholar]
- Schwarz LV, Marcon AR, Delamare APL, Echeverrigaray S. Influence of nitrogen, minerals and vitamins supplementation on honey wine production using response surface methodology. J Apic Res. 2020;60(1):1–10. doi: 10.1080/00218839.2020.1793277. [DOI] [Google Scholar]
- Sharma M, Chandel D, Shukla G. Antigenotoxicity and cytotoxic potentials of metabiotics extracted from isolated probiotic, Lactobacillus rhamnosus MD 14 on caco-2 and HT-29 human colon cancer cells. Nutr Cancer. 2020;72(1):110–119. doi: 10.1080/01635581.2019.1615514. [DOI] [PubMed] [Google Scholar]
- Singh M, Panesar PS, Marwaha SS. Studies on the suitability of kinnow fruits for the production of wine. J Food Sci Technol. 1998;35(5):455–457. [Google Scholar]
- Sinha K, Mishra NP, Singh J, Khanuja SPS. Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic applications: a review. Indian J Trad Knowl. 2004;2004(3):257–270. [Google Scholar]
- Slima B, Ktari N, Triki M, Trabelsi I, Abdeslam A, et al. Effects of probiotic strains, Lactobacillus plantarum TN8 and Pediococcus acidilactici, on microbiological and physico-chemical characteristics of beef sausages. LWT Food Sci Tech. 2018;92(2018):195–203. doi: 10.1016/j.lwt.2018.02.038. [DOI] [Google Scholar]
- Slininger PJ, Dien BS, Gorsich SW, Liu ZL. Nitrogen source and mineral optimization enhance D-xylose conversion to ethanol by the yeast Pichia stipitis NRRL Y-7124. Appl Microbiol Biotechnol. 2006;72(6):1285–1296. doi: 10.1007/s00253-006-0435-1. [DOI] [PubMed] [Google Scholar]
- Soni SK, Bansal N, Soni R. Standardization of conditions for fermentation and maturation of wine from amla (Emblica officinalis Gaertn.) Indian J Nat Prod Resour. 2009;8(4):436–444. [Google Scholar]
- Sriramaneni RN, Omar AZ, Ibrahim SM, Amirin S, Mohd ZA. Vasorelaxant effect of diterpenoid lactones from and Rographis paniculata chloroform extract on rat aortic rings. Pharmacognosy Res. 2010;2:242–246. doi: 10.4103/0974-8490.69125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarraj S, Thangam R, Sreevani V, Kaveri K, Gunasekaran P, Achiraman S, et al. ϒ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apopyosis through c-Myc suppression in MCF-7 and A549 cells. J Ethnopharmcol. 2012;141:803–809. doi: 10.1016/j.jep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Swami U, Rishi P, Soni SK. A non-conventional wine from stem of Syzygium cumini and statistical optimization of its fermentation conditions for maximum bioactive extraction. Int J Food Ferment Technol. 2016;6(1):25–34. doi: 10.5958/2277-9396.2016.00023.4. [DOI] [Google Scholar]
- Swami U, Rishi P, Soni SK. Anti-diabetic, hypolipidemic and hepato-renal protective effect of a novel fermented beverage from Syzygium cumini stem. Int J Pharm Sci. 2017;8(3):1336–1345. [Google Scholar]
- Thippeswamy G, Sheela ML, Salimath BP. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF<kappa>B and its DNA binding activity. Eur J Pharmcol. 2008;588:141–150. doi: 10.1016/j.ejphar.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Shukla S, Kishor K. Production, optimization, characterization and evaluation of antimicrobial activities in Hibiscus rosasinensis wine. Pharmacogn Phytochem. 2017;6(3):19–26. [Google Scholar]
- Upadhaya AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd.) Hook. F. and Thoms. (Guduchi)-alidation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1:112–121. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero MR, Alberto MR, De Nadra MM. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18(2):93–101. doi: 10.1016/j.foodcont.2005.08.010. [DOI] [Google Scholar]
- Vasudha S, Mishra HN. Nondairy probiotic beverages. Int Food Res J. 2013;20(1):7–15. [Google Scholar]
- Verma A, Shukla G. Probiotic Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Spargue Dawley rats. Nutr Cancer. 2013;65:84–91. doi: 10.1080/01635581.2013.741746. [DOI] [PubMed] [Google Scholar]
- Wang T, Liu YY, Wang X, Yang N, Zhu HB, Zuo PP. Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGP and NGF signalling. Acta Pharmacol Sin. 2010;31:765–774. doi: 10.1038/aps.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wet HD, Wyk BV. An ethnobotanical survey of southern African Menispermaceae. S Afr J Bot. 2008;74:2–9. doi: 10.1016/j.sajb.2007.07.001. [DOI] [Google Scholar]
- Xavier-Santosa D, Bedania R, Limad ED, Saada SMI. Impact of probiotics and prebiotics targeting metabolic syndrome. J Funct Foods. 2019;64:103666. doi: 10.1016/j.jff.2019.103666. [DOI] [Google Scholar]
- Yang S, Evens AM, Prachands SAT, Bhalla S, Devid K, et al. Diterpenoid lactone and rographolide, the active component of and Rographis paniculata. Clin Cancer Res. 2010;16:4755–4768. doi: 10.1158/1078-0432.CCR-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The presented data is available from the corresponding author on reasonable request.