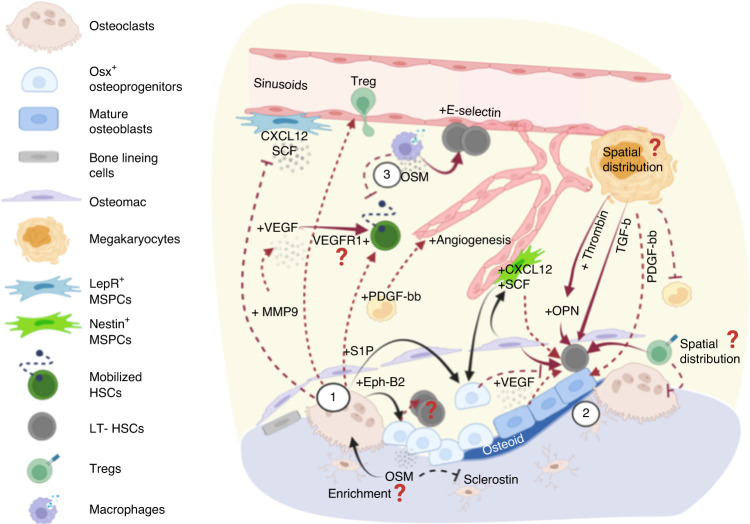
Fig. 2.
Factors involved in the reversal stage of bone remodeling and their crosstalk with the hematopoietic compartment. (1) Osteoclasts secrete several coupling factors to promote osteodifferentiation during the reversal stage of bone remodeling and that also target hematopoietic cells. S1P acts through S1P1 receptors that are highly expressed on hematopoietic cells to regulate cell trafficking, which is crucial for cell egress after treatment with mobilizing agents (e.g., G-CSF or GM-CSF). Osteoclast-derived Ephrin-B2 acts on EphB4-expressing osteolineage cells, leading to the subsequent expansion of long-term HSCs mediated via mechanisms that remain to be elucidated. Osteoclasts also recruit regulatory T cells, which may constitute immune-privileged sites that promote HSC survival. In addition, MMP9 secreted from osteoclasts modulates the bone marrow microenvironment in several ways, including the release of VEGF from extracellular matrix to promote angiogenesis and the degradation/shedding of CXCL12 and SCF to promote HSC mobilization. (2) In a bone remodeling unit, osteolineage cells, osteomacs, and megakaryocytes (MKs) work collaboratively to promote osteogenesis. Each unit produces abundant factors such as thrombin-cleaved, activated OPN, and MK-derived TGF-β, which regulate HSC dormancy. (3) Moreover, oncostatin M (OSM) secreted by osteolineage cells (e.g., MSPCs and osteoblasts) or immune cells (e.g., macrophages) plays pleiotropic roles to promote both remodeling (inducing RANKL expression) and osteodifferentiation (suppressing sclerostin expression). Importantly, OSM induces CXCL12 to inhibit cell mobilization and boost E-selectin-mediated HSC self-renewal/expansion. Overall, the reversal stage involves functionally diverse signaling pathways that promote dormancy, mobilization, and expansion. It will be insightful to understand whether HSC proliferation in such microenvironment is mediated by self-renewal-based expansion or loss of stemness, and whether active bone remodeling leads to enrichment in MK distribution, OPN/OSM concentration, and accumulation of regulatory T cells that are associated with the self-renewal potency and survival of HSCs