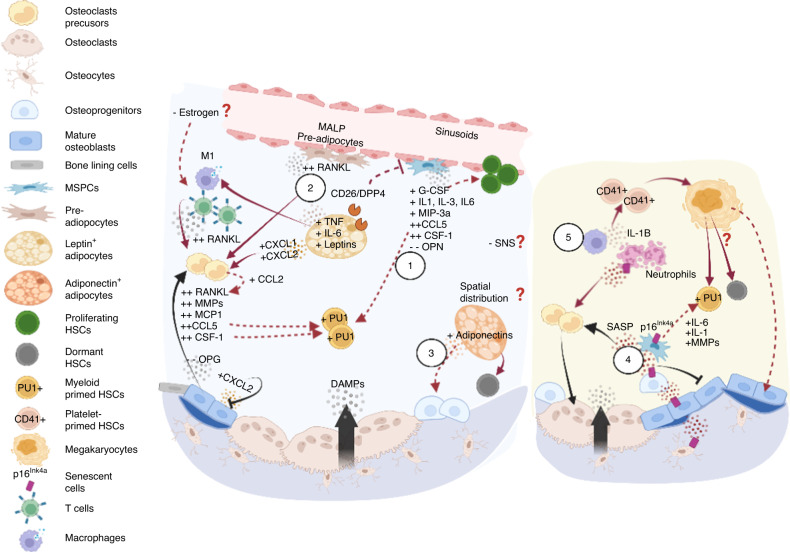
Fig. 4.
Skeletal aging and its impact on the hematopoietic compartment. Aging of osteolineage cells leads to an overall inflammatory microenvironment (left panel) and acquisition of the senescence-associated secretory phenotype (SASP, right panel). (Left 1) In general, aged osteoprogenitors employ a myeloid-promoting program, attributed to telomere dysfunction that manifests as upregulation of G-CSF, IL-3, MIP-3a levels in bone-associated stromal cells, and aging of bone cells that overexpress CSF-1, IL-1, CCL5, etc. (2) Aged MSPCs are also primed for adipocyte differentiation, synergistically promoting myelopoiesis. Specifically, the preadipocyte (Pref-1+ or MALP) population is expanded with upregulated RANKL expression. Adipocytes further secrete leptins, DPP4, IL-6, TNF, and CXCL1/CXCL2 to promote osteoclastogenesis while inhibiting osteodifferentiation, which promotes the proinflammatory immune phenotype. (3) In contrast, adiponectins promote osteoprogenitors and have been shown to protect HSCs from inflammatory insult and enhance self-renewal. Hypothetically, leptin- and adiponectin-secreting adipocytes exhibit spatial associations with bone resorption and formation sites, respectively, and may contribute to differential HSC responses. (Right 4) Multiple cell types, including osteoblasts, osteoprogenitors, osteocytes, lymphocytes, and myeloid cells, undergo senescence and are associated with the overproduction of IL-6, IL-1, MMPs and other proinflammatory cytokines that promote bone resorption and myeloid bias. (5) Reduced phagocytic capability of macrophages leads to the accumulation of senescent neutrophils, an increase in IL-1 level and induced platelet bias. Whether increased megakaryopoiesis causes myeloid bias and HSC accumulation remains unclear. Overall, aging is associated with contraction of Type H vessels, and arterioles, depleting niche factors that support lymphopoiesis and possibly negatively impacting the SNS. Although important, it remains unclear whether effects of estrogen deficiency on bone remodeling kinetics cause substantial variability in aged HSC niches between sexes