Abstract
BACKGROUND:
There is limited information regarding the true frequency of nonmedical opioid use (NMOU) among patients receiving opioid therapy for cancer pain. Data to guide patient selection for urine drug testing (UDT) as well as the timing and frequency of ordering UDT are insufficient. This study examined the frequency of abnormal UDT among patients with cancer who underwent random UDT and their characteristics.
METHODS:
Demographic and clinical information for patients with cancer who underwent random UDT were retrospectively reviewed and compared with a historical cohort that underwent targeted UDT. Random UDT was ordered regardless of a patient’s risk potential for NMOU. Targeted UDT was ordered on the basis of a physician’s estimation of a patient’s risk for NMOU.
RESULTS:
In all, 552 of 573 eligible patients (96%) underwent random UDT. Among these patients, 130 (24%) had 1 or more abnormal results; 38 of the 88 patients (43%) who underwent targeted UDT had 1 or more abnormal results. When marijuana was excluded, 15% of the random group and 37% of the targeted group had abnormal UDT findings (P < .001). It took a shorter time from the initial consultation to detect 1 or more abnormalities with the random test than the targeted test (median, 130 vs 274 days; P = .02). Abnormal random UDT was independently associated with younger age (P < .0001), male sex (P = .03), Cut Down, Annoyed, Guilty, and Eye Opener–Adapted to Include Drugs positivity (P = .001), and higher Edmonton Symptom Assessment System anxiety (P = .01).
CONCLUSIONS:
Approximately 1 in 4 patients receiving opioids for cancer pain at a supportive care clinic who underwent random UDT had 1 or more abnormalities. Random UDT detected abnormalities earlier than the targeted test. These findings suggest that random UDT is justified among patients with cancer pain.
Keywords: cancer pain, opioid, random, targeted, urine drug test
INTRODUCTION
Opioids are the main treatment for cancer-related pain,1,2 but nonmedical opioid use (NMOU) presents a significant challenge for health care providers. Clinicians need to maintain a complex balance between ensuring legitimate patient access to opioids and minimizing NMOU. Regrettably, no definitive test or pathognomonic sign exists to help to predict which patients will be adherent in a therapeutic trial of opioids for pain. Urine drug testing (UDT) is an effective risk monitoring tool during chronic opioid therapy3,4 because it provides support for clinicians during therapeutic decision making and has been endorsed in numerous opioid prescribing guidelines for chronic nonmalignant pain.5–7 On the other hand, there is very limited evidence to guide the integration of UDT into routine cancer pain management,8,9 and no universally approved standardized protocol currently exists. Usually, patients are selected to undergo UDT on the basis of their level of risk for NMOU (targeted patient selection),10 but sometimes they may undergo the test regardless of their risk for NMOU (random patient selection).8
Our supportive care center implemented a policy to randomly test outpatients on opioids regardless of their risk profile. In a preliminary study involving a sample of 212 patients who underwent random UDT, we found that 1 in 4 patients had abnormal UDT concerning for NMOU. Even when marijuana was excluded from the list of urine abnormalities, the rate still remained considerably high at 1 in 6 patients.11 In this follow-up study, we further examined the frequency of UDT abnormalities in a larger sample of patients with cancer who underwent random UDT and compared them with a historical cohort of patients with cancer who underwent targeted UDT. We also explored factors associated with marijuana use among patients receiving opioids for cancer pain.
MATERIALS AND METHODS
Study Participants and Procedure
We reviewed electronic medical records of patients seen at MD Anderson’s outpatient supportive care clinic between April 2017 and October 2019. Patients were randomly selected daily to undergo UDT regardless of their risk profile for NMOU (random group). Eligible patients were aged ≥18 years, had a diagnosis of cancer, and were receiving chronic opioid therapy (defined as the treatment of pain with opioids for ≥7 days12). This random cohort was compared with a previous cohort of patients with cancer with similar characteristics who underwent UDT on the basis of their elevated risk for NMOU as determined by the clinician (targeted group) in the same clinic between March 1, 2015, and February 31, 2017.11 The institutional review board at the University of Texas MD Anderson Cancer Center approved the study protocol a priori.
Data Collection
Patients’ baseline demographic and clinical characteristics at the initial consultation visit and on the day when UDT was first ordered were obtained. These included the following: age, sex, race, cancer diagnosis, cancer stage, educational status, insurance status, morphine equivalent daily dose, Edmonton Symptom Assessment System (ESAS) score,13–15 performance status, Memorial Delirium Assessment Scale score,16,17 Cut Down, Annoyed, Guilty, and Eye Opener–Adapted to Include Drugs (CAGE-AID) score,18–20 Screener and Opioid Assessment for Patients with Pain (SOAPP) score,21,22 and prescribed opioids that patients were receiving on the day when UDT was ordered. Patient information regarding opioid intake at the time of urine testing was obtained to assist in the interpretation of UDT results. The types of UDT abnormalities were recorded. An abnormal UDT result was defined as any of the following: the unexpected absence of a prescribed opioid in the urine, the unexpected presence of an unprescribed opioid in the urine, or the presence of an illicit drug (eg, marijuana, cocaine, or heroin). For patients who had more than 1 UDT, information on only the first test was obtained for the study.
Clinic Process and Instruments
As part of an opioid safety program at the supportive care clinic, all patients receiving chronic opioid therapy are systematically screened with risk assessment tools such as CAGE-AID, SOAPP, and a prescription drug monitoring program database to determine their level of risk. High-risk patients are monitored more closely on an ongoing basis; this includes close observation of certain behavioral patterns suggestive of NMOU.23 Patients may be particularly asked to undergo targeted UDT on the basis of their level of risk as determined by the physician. Alternatively, patients may be randomly selected to undergo UDT regardless of their level of risk. At the beginning of each clinic day, the charge nurse will randomly preselect approximately 15% of all scheduled follow-up patients for UDT by using a randomly generated computer system. Occasionally, a preselected patient may not undergo the test for logistic reasons such as limited clinic staff, busy clinical schedules, staff reassignments, natural disasters interrupting the clinic flow, and other situations that put an undue clinical burden on the workforce on that day. Other reasons that the test may not be performed include a high patient symptom burden, clinician errors in ordering the test, no opioid intake, and patient refusal to give a sample (Fig. 1).
Figure 1.
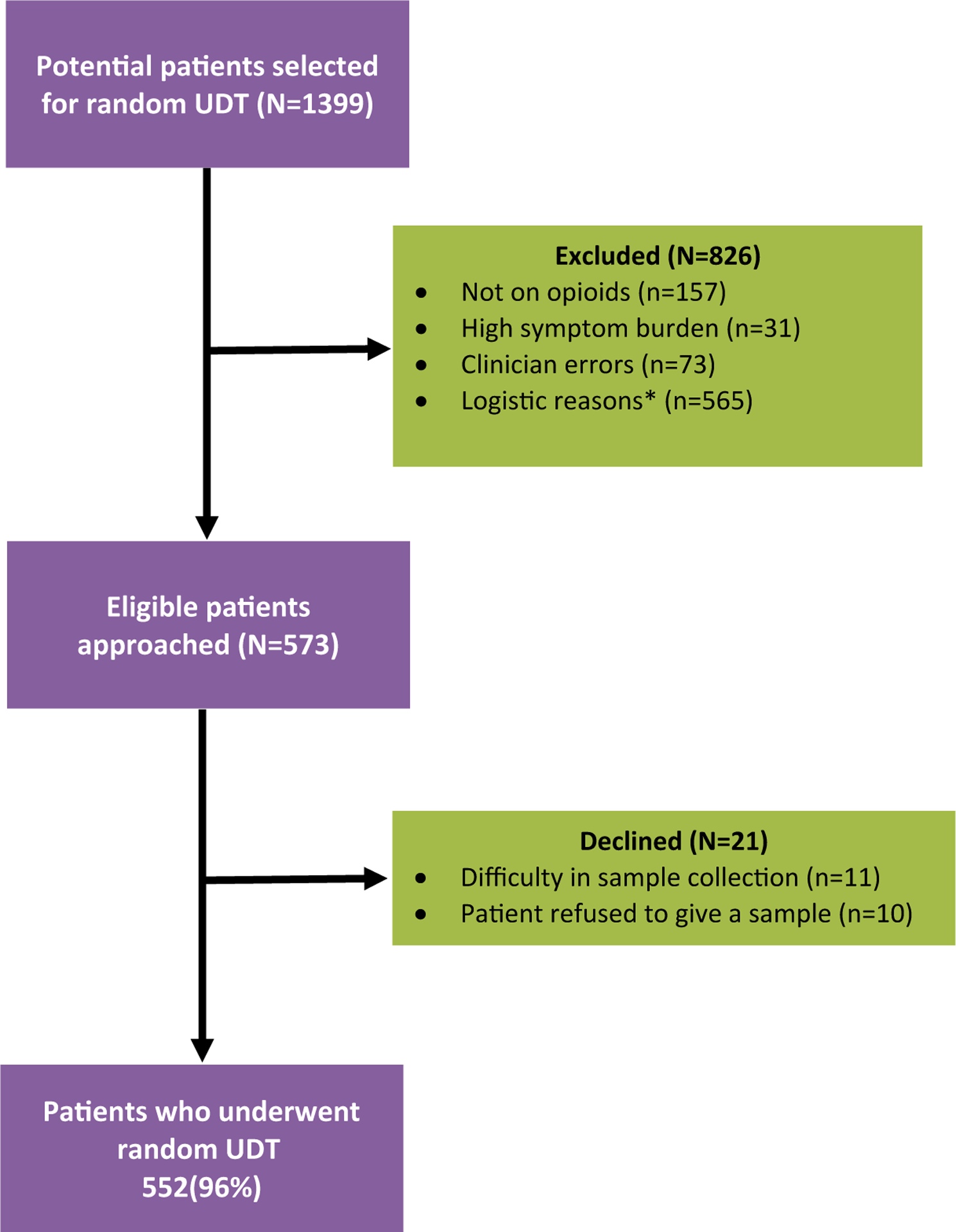
Study participant flow chart. *Examples include limited clinic staff, busy clinical schedules, staff reassignments, and natural disasters interrupting the clinic workflow. UDT indicates urine drug testing.
When NMOU is detected, patients will receive specific care provided by a special interdisciplinary team consisting of a physician, a registered nurse, a pharmacist, a psychologist/counselor, a social worker, and a patient advocate; this mainly entails extensive opioid education, risk mitigation, and harm reduction strategies. Details of this intervention have been previously published by our group.24
Urine Drug Testing
Generally, 2 main types of UDT are used in clinical practice. The screening tests or immunoassays use antibodies to detect the presence of a particular drug or metabolite in a urine sample. The confirmatory tests or laboratory-based specific drug identification tests, such as gas chromatography–mass spectrometry, liquid chromatography–mass spectrometry, and liquid chromatography–tandem mass spectrometry, use techniques that separate the drug or drug metabolite from other analytes (chromatography) and then identify it on the basis of its molecular structure and properties (mass spectrometry).10,25,26 Each test has its own advantages and disadvantages.26,27 The test used in this study is called Pain Clinic Drug Screen, Urine, and it is processed at Mayo Medical Laboratories.28 It is designed to use immunoassay testing in screening for the following drugs with their cutoff concentrations: alcohol (30 mg/dL), amphetamines (500 ng/mL), barbiturates (200 ng/mL), benzodiazepines (200 ng/mL), cocaine (benzoylecgonine-cocaine metabolite; 150 ng/mL), ethanol (30 mg/dL), methadone (150 ng/mL), opiates (300 ng/mL), phencyclidine (25 ng/mL), and tetrahydrocannabinol carboxylic acid (20 ng/mL). A positive opiate immunoassay test usually refers to the presence of an unspecified opioid in the urine. All positive screening results are confirmed by gas chromatography–mass spectrometry, gas chromatography–flame ionization detection, or liquid chromatography–tandem mass spec-trometry before a final positive result is reported.
Statistical Analysis
Descriptive statistics such as frequencies and percentages for categorical data and medians and interquartile ranges for continuous variables were provided to summarize the results. Associations between categorical variables were assessed with a chi-square test or Fisher exact test when appropriate. Continuous variables were compared between 2 groups with the Wilcoxon rank sum test. Univariate and multicovariate logistic regression analyses were used to explore the demographics and clinical factors associated with abnormal UDT findings as well as the presence of marijuana in urine. All computations were performed with SAS 9.4 and TIBCO Spotfire S+ 8.2.
RESULTS
Figure 1 shows study participant accrual information. There were 1399 potential patients selected for random UDT, and 552 eligible patients underwent the test. Table 1 provides information on the demographic and clinical characteristics of patients who underwent both random and targeted UDT. The median age was 59 years in the random cohort and 53 years in the targeted cohort. The majority of the patients in both cohorts were female and White and had advanced cancer. Table 2 shows the rate of UDT abnormalities among patients who underwent random and targeted testing. 130 of the 552 patients in the random group (24%) and 38 of the 88 patients in the targeted group (43%) had 1 or more abnormalities. Similarly, 19 of the 552 patients (3%) and 10 of the 88 patients (11%) had 2 or more abnormalities in the random and targeted groups, respectively. When marijuana was excluded from the list of abnormal results, 15% of the random group and 37% of the targeted group had abnormal UDT findings (P < .001).
TABLE 1.
Demographic and Clinical Characteristics of Random and Targeted Urine Drug Testing Cohorts
| Random Patients | Target Patientsa | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Total (N = 552) | Abnormal (n = 130 [24%]) | Normal (n = 422 [76%]) | P b | Total (N = 88) | Abnormal (n = 38 [43%]) | Normal (n = 50 [57%]) | P b |
| Age, median (IQR), y | 59 (50–68) | 56.5 (50–68) | 61 (52–69) | <.001 | 53 (40–61) | 51 (39–60) | 56 (41–62) | .35 |
| Sex, No. (%) | ||||||||
| Female | 301 (55) | 58 (45) | 243 (58) | .01 | 45 (51) | 20 (53) | 25 (50) | .81 |
| Race, No. (%) | ||||||||
| Black | 104 (19) | 27 (21) | 77 (18) | .44 | 28 (32) | 14 (37) | 14 (28) | .80 |
| Hispanic | 50 (9) | 9 (7) | 41 (10) | 7 (8) | 3 (8) | 4 (8) | ||
| Other | 24 (4) | 3 (2) | 21 (5) | 4 (4) | 1 (3) | 3 (6) | ||
| White | 374 (68) | 91 (70) | 283 (67) | 49 (56) | 20 (52) | 29 (58) | ||
| Insurance status, No. (%) | ||||||||
| Medicaid or indigent | 26 (5) | 13 (10) | 13 (3) | .01 | 9 (10) | 3 (8) | 6 (12) | .30 |
| Medicare | 226 (41) | 44 (34) | 182 (43) | 28 (32) | 16 (42) | 12 (24) | ||
| Otherc | 35 (6) | 7 (5) | 28 (7) | 8 (9) | 2 (5) | 6 (12) | ||
| Private insurance | 265 (48) | 66 (51) | 199 (47) | 43 (49) | 17 (45) | 26 (52) | ||
| Cancer type, No. (%) | ||||||||
| Breast | 94 (17) | 12 (9) | 82 (19) | .11 | 18 (21) | 10 (26) | 8 (16) | .76 |
| Gastrointestinal | 93 (17) | 27 (21) | 66 (16) | 17 (19) | 5 (13) | 12 (24) | ||
| Genitourinary | 83 (15) | 20 (15) | 63 (15) | 7 (8) | 4 (11) | 3 (6) | ||
| Gynecological | 44 (8) | 14 (11) | 30 (7) | 8 (9) | 4 (11) | 4 (8) | ||
| Head and neck | 63 (11) | 16 (12) | 47 (11) | 21 (24) | 8 (21) | 13 (26) | ||
| Leukemia/lymphoma | 26 (5) | 9 (7) | 17 (4) | 2 (2) | 1 (3) | 1 (2) | ||
| Other | 58 (11) | 15 (12) | 43 (10) | 8 (9) | 4 (10) | 4 (8) | ||
| Thoracic | 91 (16) | 17 (13) | 74 (18) | 7 (8) | 2 (5) | 5 (10) | ||
| Cancer stage, No. (%) | ||||||||
| Advancedd | 484 (88) | 116 (89) | 368 (87) | .79 | 71 (81) | 34 (90) | 37 (74) | .20 |
| Early | 46 (8) | 9 (7) | 37 (9) | 10 (11) | 2 (5) | 8 (16) | ||
| NED | 22 (4) | 5 (4) | 17 (4) | 7 (8) | 2 (5) | 5 (10) | ||
| CAGE-AID status, No. (%) | <.001 | .96 | ||||||
| Positive | 82 (15) | 34 (27) | 48 (12) | 26 (30) | 11 (31) | 15 (30) | ||
| Negative | 459 (85) | 93 (73) | 366 (88) | 60 (70) | 25 (69) | 35 (70) | ||
| Unknown | 11 | 2 | ||||||
| MEDD, median (IQR), mg/d | 57 (24–120) | 75 (37.5–150) | 50 (20–115) | <.001 | 105 (50–210) | 90 (50–250) | 113 (45–200) | .95 |
| SOAPP status, No. (%) | .05 | 1.00 | ||||||
| Positive | 85 (26) | 24 (36) | 61 (24) | 11 (50) | 5 (56) | 6 (46) | ||
| Negative | 238 (74) | 43 (64) | 195 (76) | 11 (50) | 4 (44) | 7 (54) | ||
| Unknown | 229 | 66 | ||||||
| ESAS pain, median (IQR) | 4 (3–6) | 5 (3–7) | 4 (2–6) | .008 | 7 (4–8) | 7 (4–9) | 7 (4–8) | .68 |
Abbreviations: CAGE-AID, Cut Down, Annoyed, Guilty, and Eye Opener–Adapted to Include Drugs; ESAS, Edmonton Symptom Assessment Scale; IQR, interquartile range; MEDD, morphine equivalent daily dose; NED, no evidence of disease; SOAPP, Screener and Opioid Assessment for Patients With Pain.
Historical cohort from a previous study.11
A P value <.05 indicates statistical significance. Values in bold are statistically significant
Includes self-pay.
Includes metastatic, locally advanced, relapsed, and/or refractory disease.
TABLE 2.
Frequency of Abnormal UDT Among Patients Who Underwent Random and Targeted Testing
| Frequency (%) | |||
|---|---|---|---|
| Random Cohort | Targeted Cohort | P a | |
| All patients with ≥1 UDT abnormality | 130/552 (24) | 38/88 (43) | <.001 |
| Patients with ≥1 UDT abnormality excluding marijuana | 76/498 (15) | 29/79 (37) | <.001 |
Abbreviation: UDT, urine drug testing.
Pearson chi-square values.
It took a significantly shorter time from the initial consultation for UDT abnormalities to be detected in the random group in comparison with the targeted group (median, 130 vs 274 days; P = .02). Table 3 summarizes the regression analysis models of factors associated with any abnormal UDT finding and marijuana use in the random cohort. In a multivariate analysis, the odds ratio for any abnormal UDT was 1.62 for males versus females (P = .03), 2.51 for CAGE-AID positivity versus CAGE-AID negativity (P = .007), 0.96 per 1-year increase in age (P < .0001), and 1.11 per 1-point increase in the ESAS anxiety score (P = .01). Similarly, male sex, younger age, CAGE-AID positivity, and higher ESAS anxiety scores were independently associated with marijuana use.
TABLE 3.
Univariate and Multivariate Regression Analyses for Factors Associated With Abnormal Random Urine Drug Testing
| Any Abnormality | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Covariate | OR (95% CI) | P | OR (95% CI) | P |
| Sex (male vs female) | 1.69 (1.13–2.50) | .01 | 1.62 (1.05–2.50) | .03 |
| Age | 0.97 (0.95–0.98) | <.001 | 0.96 (0.95–0.98) | <.001 |
| CAGE-AID (positive vs negative) | 2.79 (1.70–4.57) | <.001 | 2.51 (1.47–4.27) | .001 |
| ESAS anxiety | 1.12 (1.04–1.20) | .003 | 1.11 (1.03–1.20) | .01 |
| SOAPP (positive vs negative) | 1.78 (1.00–3.16) | .05 | ||
| Race (Black vs White) | 1.09 (0.66–1.79) | .14 | ||
| MEDD (mg/d) | 1.00 (1.00–1.00) | .01 | ||
| MDAS | 0.83 (0.70–0.99) | .04 | ||
| ESAS pain | 1.11 (1.02–1.20) | .01 | ||
| ESAS nausea | 1.08 (1.00–1.17) | .05 | ||
| ESAS sleep | 1.08 (1.00–1.16) | .06 | ||
| ESAS financial distress | 1.07 (1.01–1.14) | .03 | ||
| Marijuana Abnormality | ||||
| Univariate Analysis | Multivariate Analysis | |||
| Covariate | OR (95% CI) | P | OR (95% CI) | P |
| Sex (male vs female) | 2.48 (1.43–4.28) | .001 | 2.14 (1.17–3.93) | .01 |
| Age | 0.96 (0.94–0.98) | <.001 | 0.96 (0.94–0.98) | <.001 |
| CAGE-AID (positive vs negative) | 4.72 (2.64–8.45) | <.001 | 3.96 (2.10–7.47) | <.001 |
| ESAS anxiety | 1.14 (1.04–1.25) | .01 | 1.12 (1.01–1.24) | .03 |
| SOAPP (positive vs negative) | 3.19 (1.48–6.84) | .003 | ||
| MEDD (mg/d) | 1.00 (1.00–1.00) | .07 | ||
| MDAS | 0.84 (0.66–1.07) | .16 | ||
| ESAS nausea | 1.08 (0.97–1.19) | .15 | ||
| ESAS financial distress | 1.07 (0.99–1.16) | .11 | ||
Abbreviations: CAGE-AID, Cut Down, Annoyed, Guilty, and Eye Opener–Adapted to Include Drugs; CI, confidence interval; ESAS, Edmonton Symptom Assessment Scale; MDAS, Memorial Delirium Assessment Scale; MEDD, morphine equivalent daily dose; OR, odds ratio; SOAPP, Screener and Opioid Assessment for Patients With Pain.
Variables with a P value <.2 were considered in building the multicovariate model. Abnormal random urine drug testing was defined as 1 or more urine abnormalities.
Figure 2 summarizes the types and distributions of UDT abnormalities in both cohorts. Hydrocodone was the most frequent opioid and marijuana was the most frequent nonopioid substance detected among the abnormal tests.
Figure 2.
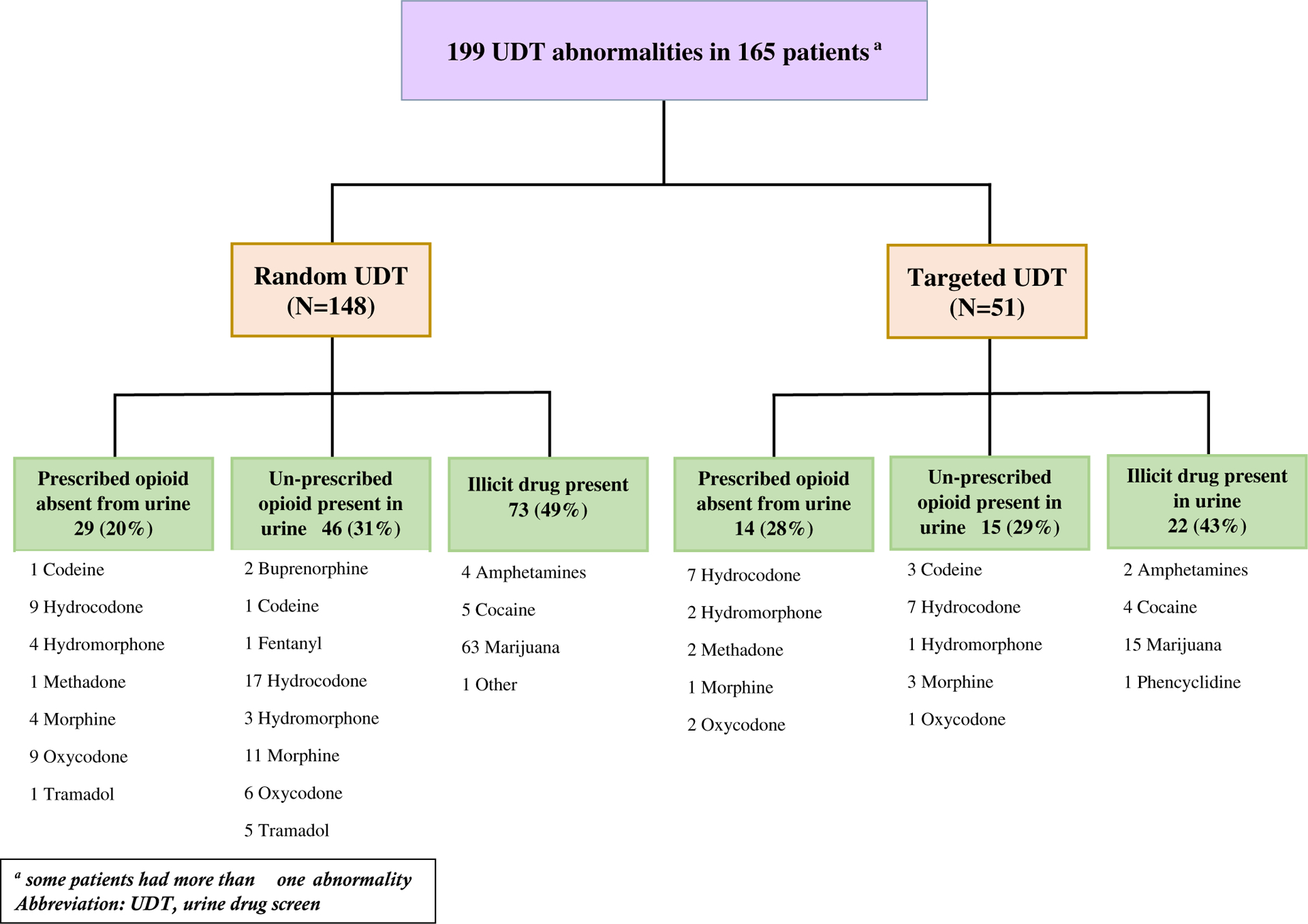
Types and distributions of UDT abnormalities in the random and targeted cohorts. aSome patients had more than 1 abnormality. UDT indicates urine drug testing.
DISCUSSION
In this study, we found that approximately 1 in every 4 patients receiving chronic opioid therapy for cancer pain had abnormal UDT concerning for NMOU. Even when marijuana was excluded from the list of urine abnormalities because of the contentious views surrounding its classification as a controlled substance, the rate of NMOU still remained considerably high at 1 in 7 patients. This supports emerging evidence that, in contrast to previous perceptions, patients with cancer may also be at risk for NMOU.3,29 According to an integrative review by Carmichael et al,30 at least 1 in 5 patients with cancer might be at risk for opioid use disorder. In another study, 18% of patients with advanced cancer were clinically suspected by their clinicians to cope chemically with opioids.20 Our study is innovative in that it assessed the frequency of UDT abnormalities in a random sample of patients who were receiving opioids for cancer pain. Other studies reporting on the frequency of abnormal UDT in patients with cancer were conducted with a purposefully selected sample of patients with an already known elevated risk of NMOU, and this raises questions about a potential selection bias. Such studies may, therefore, not be reflective of the true frequency in this patient population.18,23,31–33 We acknowledge that the cohort in our study consisted of patients referred to a supportive care clinic with a relatively high level of symptom burden and distress and may, therefore, be more inclined to have a higher risk for NMOU than the average oncology patient.
Random UDT detected abnormalities earlier than targeted UDT, and this suggests that random UDT may be a very efficient way to aid in early patient identification; support therapeutic decision making; and avoid unintended consequences such as NMOU, accidental overdose, or death. Currently, no standardized or universally accepted guideline regarding the manner of UDT ordering in patients with cancer exists. Different recommendations proposed in the literature mainly have been made for patients with chronic noncancer pain.10,34–37 This study provides a key step in our efforts to better understand and define the most efficient manner of ordering UDT among patients with cancer. This process needs to be further investigated to better determine the most optimal timing and frequency of UDT ordering. The cost-effectiveness of these tests38,39 should be considered carefully to prevent any undue financial40,41 or logistic burden on patients.
The fact that it took a relatively long time to detect NMOU when the test was ordered for only high-risk patients raises concerns about the possibility of underdetection or missed opportunities for timely patient identification, especially among individuals who may appear to be at minimal risk for NMOU and are, therefore, likely to fly under the radar. In addition, some patients who initially exhibit opioid-adherent behavior can subtly transition into a maladaptive pattern of NMOU later during their opioid therapy, and these can easily be missed when only risk-based UDT ordering is practiced. Moreover, overreliance on validated risk assessment tools may not be always be adequate because they are mainly based on patient self-report, which is not always reliable.42 All these factors further underscore the value of random testing in clinical practice.
This study found that younger age, male sex, CAGE-AID positivity, and higher anxiety were independently associated with abnormal UDT. The findings are generally consistent with numerous other studies that have constantly identified these risk factors as strong predictors of NMOU.18,43,44 The coexistence of common psychiatric conditions such as anxiety disorders in patients with a history of substance use disorder is extremely high.45 Notably, the same risk factors were associated with marijuana use. Also, there was a trend for an association between increasing opioid use and marijuana use. This suggests that increasing opioid use among marijuana users either might indicate a predilection for NMOU or is an indication of suboptimal pain control. Further studies are needed to investigate these findings. The presence of marijuana in the urine may be of limited importance mainly because its classification as a controlled substance is currently debatable and the general perception continues to evolve. Although it remains federally prohibited, there are numerous ongoing efforts to legalize its use in many states.46 At the time of this study, medical marijuana was not legally approved for use in the state of Texas except for a few clinical conditions.47
Although there was a trend for an association between SOAPP positivity and abnormal UDT in the univariate analysis, it was unexpectedly nonpredictive of UDT abnormalities. The reason for this is unclear, but it might likely be due to a considerable number of patients without information regarding their SOAPP status. Approximately 229 of the patients in the random cohort (41%) had unavailable SOAPP questionnaire information. Future studies involving a bigger sample size with more SOAPP questionnaire information will be needed to better evaluate the association between SOAPP positivity and UDT abnormality.
One limitation of the study is the retrospective design. Also, the study was conducted among patients with cancer who had a relatively high level of symptom burden and distress and a potentially higher level of NMOU. The results may, therefore, not be generally applicable to other cancer patient populations receiving opioid therapy. Lastly, a normal UDT result does not always rule out NMOU. One of the most common forms of NMOU is taking prescribed opioids more frequently than directed.48 Unfortunately, such behavior cannot be detected by UDT; hence, such patients may have normal UDT but still be using the opioid in an excessive or maladaptive manner. It is possible that the frequency of NMOU was higher than what we found in our study. The therapeutic decision-making process surrounding opioid therapy should not be based solely on UDT, and more research is needed.
In conclusion, approximately 1 in 4 patients receiving opioids for cancer pain who underwent random UDT had abnormalities concerning for NMOU. The random test detected abnormalities earlier than the targeted test. These findings suggest that random UDT is justified among patients receiving opioids for cancer pain. Further studies are needed to ascertain these observations in different cohorts and clinical settings to better characterize its use in cancer pain management.
FUNDING SUPPORT
This work was supported by the National Institutes of Health through Award Number 1UL1TR003167.
CONFLICT OF INTEREST DISCLOSURES
Sriram Yennurajalingam reports grants from Helsinn Healthcare and Genentech outside the submitted work. Eduardo Bruera reports grants from Helsinn Healthcare outside the submitted work. The other authors made no disclosures.
REFERENCES
- 1.Stjernsward J WHO cancer pain relief programme. Cancer Surv. 1988;7:195–208. [PubMed] [Google Scholar]
- 2.Stjernsward J, Colleau SM, Ventafridda V. The World Health Organization Cancer Pain and Palliative Care Program. Past, present, and future. J Pain Symptom Manage. 1996;12:65–72. [DOI] [PubMed] [Google Scholar]
- 3.Passik SD, Portenoy RK, Ricketts PL. Substance abuse issues in cancer patients. Part 1: prevalence and diagnosis. Oncology (Williston Park). 1998;12:517–521. [PubMed] [Google Scholar]
- 4.Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18:S76–S82. [DOI] [PubMed] [Google Scholar]
- 5.Chou R 2009 clinical guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469–477. [PubMed] [Google Scholar]
- 6.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:3325–3345. [DOI] [PubMed] [Google Scholar]
- 8.Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123–143. [PubMed] [Google Scholar]
- 9.Arthur JA. Urine drug testing in cancer pain management. Oncologist. 2020;25:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen GT, Burton AW, Schade CM, et al. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15:ES119–ES133. [PubMed] [Google Scholar]
- 11.Arthur J, Lu Z, Nguyen K, et al. Random vs targeted urine drug testing among patients undergoing long-term opioid treatment for cancer pain. JAMA Oncol. 2020;6:580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anghelescu DL, Ehrentraut JH, Faughnan LG. Opioid misuse and abuse: risk assessment and management in patients with cancer pain. J Natl Compr Canc Netw. 2013;11:1023–1031. [DOI] [PubMed] [Google Scholar]
- 13.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 14.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. [DOI] [PubMed] [Google Scholar]
- 15.Philip J, Smith WB, Craft P, et al. Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer. 1998;6:539–541. [DOI] [PubMed] [Google Scholar]
- 16.Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997;13:128–137. [DOI] [PubMed] [Google Scholar]
- 17.Fadul N, Kaur G, Zhang T, et al. Evaluation of the Memorial Delirium Assessment Scale (MDAS) for the screening of delirium by means of simulated cases by palliative care health professionals. Support Care Cancer. 2007;15:1271–1276. [DOI] [PubMed] [Google Scholar]
- 18.Childers JW, King LA, Arnold RM. Chronic pain and risk factors for opioid misuse in a palliative care clinic. Am J Hosp Palliat Care. 2015;32:654–659. [DOI] [PubMed] [Google Scholar]
- 19.Dev R, Parsons HA, Palla S, et al. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer. 2011;117:4551–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon JH, Tanco K, Park JC, et al. Frequency, predictors, and medical record documentation of chemical coping among advanced cancer patients. Oncologist. 2015;20:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler SF, Budman SH, Fernandez K, et al. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. [DOI] [PubMed] [Google Scholar]
- 22.Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10:1426–1433. [DOI] [PubMed] [Google Scholar]
- 23.Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122:3732–3739. [DOI] [PubMed] [Google Scholar]
- 24.Arthur J, Edwards T, Reddy S, et al. Outcomes of a specialized interdisciplinary approach for patients with cancer with aberrant opioid-related behavior. Oncologist. 2018;23:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnani B, Kwong T. Urine drug testing for pain management. Clin Lab Med. 2012;32:379–390. [DOI] [PubMed] [Google Scholar]
- 26.Zacher JL, Givone DM. False-positive urine opiate screening associated with fluoroquinolone use. Ann Pharmacother. 2004;38:1525–1528. [DOI] [PubMed] [Google Scholar]
- 27.Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography–tandem mass spectrometry versus immunoassay drug testing in pain patients. Pain Physician. 2010;13:273–281. [PubMed] [Google Scholar]
- 28.Mayo Clinic Laboratories. Pain Clinic Drug Screen, Urine. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/36071. Accessed April 23, 2020.
- 29.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael AN, Morgan L, Del Fabbro E. Identifying and assessing the risk of opioid abuse in patients with cancer: an integrative review. Subst Abuse Rehabil. 2016;7:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyyalagunta D, Bruera E, Engle MP, et al. Compliance with opioid therapy: distinguishing clinical characteristics and demographics among patients with cancer pain. Pain Med. 2018;19:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barclay JS, Owens JE, Blackhall LJ. Screening for substance abuse risk in cancer patients using the Opioid Risk Tool and urine drug screen. Support Care Cancer. 2014;22:1883–1888. [DOI] [PubMed] [Google Scholar]
- 33.Rauenzahn S, Sima A, Cassel B, et al. Urine drug screen findings among ambulatory oncology patients in a supportive care clinic. Support Care Cancer. 2017;25:1859–1864. [DOI] [PubMed] [Google Scholar]
- 34.Manchikanti L, Kaye AM, Knezevic NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3–S92. [PubMed] [Google Scholar]
- 35.Peppin JF, Passik SD, Couto JE, et al. Recommendations for urine drug monitoring as a component of opioid therapy in the treatment of chronic pain. Pain Med. 2012;13:886–896. [DOI] [PubMed] [Google Scholar]
- 36.Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed April 23, 2020.
- 37.Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melanson SEF, Petrides AK. Economics of pain management testing. J Appl Lab Med. 2018;2:587–597. [DOI] [PubMed] [Google Scholar]
- 39.Hammett-Stabler CA, Pesce AJ, Cannon DJ. Urine drug screening in the medical setting. Clin Chim Acta. 2002;315:125–135. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert JW, Wheeler GR, Mick GE, et al. Urine drug testing in the treatment of chronic noncancer pain in a Kentucky private neuroscience practice: the potential effect of Medicare benefit changes in Kentucky. Pain Physician. 2010;13:187–194. [PubMed] [Google Scholar]
- 41.Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent Medicare policy changes in Kentucky. Pain Physician. 2010;13:167–186. [PubMed] [Google Scholar]
- 42.Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15:184–191. [DOI] [PubMed] [Google Scholar]
- 43.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432–442. [DOI] [PubMed] [Google Scholar]
- 44.Edlund MJ, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. [DOI] [PubMed] [Google Scholar]
- 45.Khantzian EJ, Treece C. DSM-III psychiatric diagnosis of narcotic addicts. Recent findings. Arch Gen Psychiatry. 1985;42:1067–1071. [DOI] [PubMed] [Google Scholar]
- 46.Pacula RL, Smart R. Medical marijuana and marijuana legalization. Annu Rev Clin Psychol. 2017;13:397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birdsall SM, Birdsall TC, Tims LA. The use of medical marijuana in cancer. Curr Oncol Rep. 2016;18:40. [DOI] [PubMed] [Google Scholar]
- 48.Arthur J, Bruera E. Balancing opioid analgesia with the risk of nonmedical opioid use in patients with cancer. Nat Rev Clin Oncol. 2019;16:213–226. [DOI] [PubMed] [Google Scholar]


