Figure 2. Analysis of NID2 - HMCN1 binding by co-precipitation.
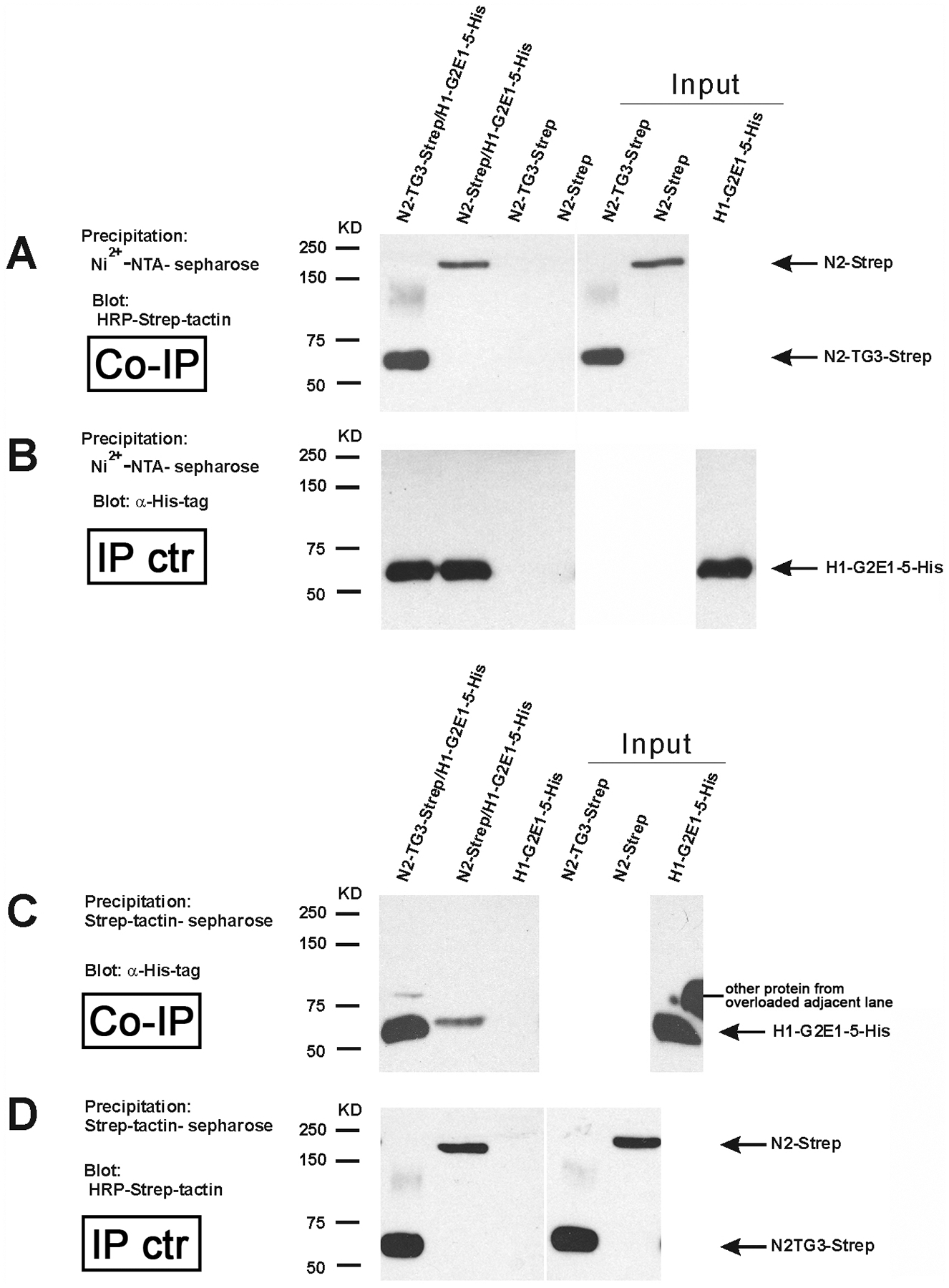
(A,B) Conditioned medium of cultured HEK293-EBNA cells transfected with NID2-Strep/HMCN1-G2E1-5-His6 or NID2-TG3-Strep/HMCN1-G2E1-5-His6 was precipitated by Ni2+-NTA sepharose and subjected to immunoblotting to detect co-precipitated Strep-tagged proteins with HRP-Strep-tactin (A) or, as control of the precipitation, His6-tagged proteins with anti-His6-tag antibodies (B) (lanes 1 and 2). As negative control, conditioned medium from cultured HEK293-EBNA cells transfected with NID2-strep or NID2-TG3-strep alone was precipitated by Ni2+-NTA sepharose and detected by immunoblotting (lanes 3 and 4).
(C,D) For the reverse co-precipitation, conditioned medium of cultured HEK293-EBNA cells transfected with NID2-Strep/HMCN1-G2E1-5-His or N2-TG3-Strep/HMCN1-G2E1-5-His was precipitated by Strep-tactin-sepharose and subjected to immunoblotting to detect co-precipitated His6-tagged proteins with anti-His6-tag antibodies (C) or, as control of the precipitation, Strep-tagged proteins with HRP-Strep-tactin (D) (lanes 1 and 2). As negative control, conditioned medium from cultured HEK293-EBNA cells transfected with HMCN1-G2E1-5-His6 alone was precipitated Strep-tactin-sepharose and detected by immunoblotting (lane 3).
Lanes 5–7 of (A-B) and 4–6 of (C,D) are direct loadings of the indicated proteins (50% of input).
Abbreviations: Co-IP, co-immunoprecipitation; IP ctr, immunoprecipitation control.
