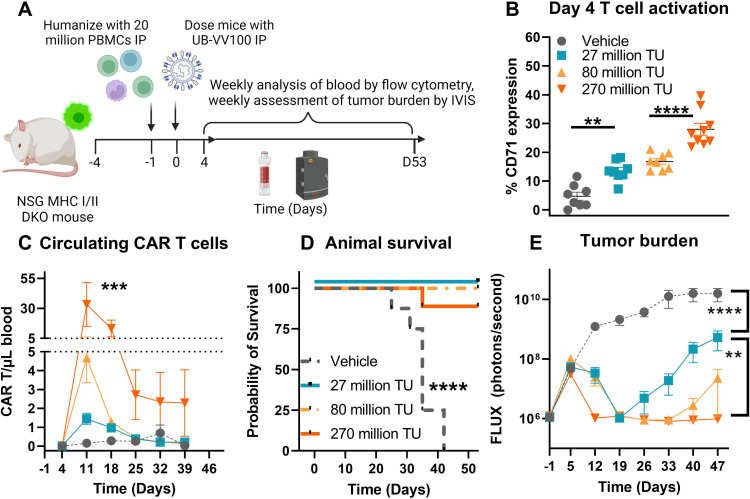
Figure 7.
UB-VV100 treatment results in in vivo transduction of CAR T cells and clearance of Nalm-6 tumor. (A) Animals were engrafted with Nalm-6 tumor on study day −4 via tail vein injection, humanized with 20E+06 PBMCs on day −1 via intraperitoneal injection, and treated with 27 million, 80 million or 270 million TU of UB-VV100 on study day 0 via intraperitoneal injection. (B) T-cell activation was assessed on day 4 by flow cytometry. **P<0.01, ****P<0.0001, one-way ANOVA, Tukey’s multiple comparison test between the indicated groups. (C) Circulating CAR T cells were enumerated by flow cytometry surface staining against FMC63. ***P<0.001, one-way ANOVA for day 11. n=7–9 per group per time point. (D) Animal survival was evaluated during the study. (E) Average tumor burden as assessed by bioluminescent imaging. (F) Heatmap of bioluminescent imaging data overlayed with mouse images. The last observed in-life photon flux data were reported for deceased animals at the indicated time points. **P<0.01, ****P<0.0001, two-way ANOVA, multiple comparisons between the indicated UB-VV100 dose level across the entire observation period. n=7–9 per group, ****P<0.0001, Mantel-Cox test. ANOVA, analysis of variance; CAR, chimeric antigen receptor; IVIS, In Vivo Imaging System; PBMC, peripheral blood mononuclear cell; TU, transducing unit.