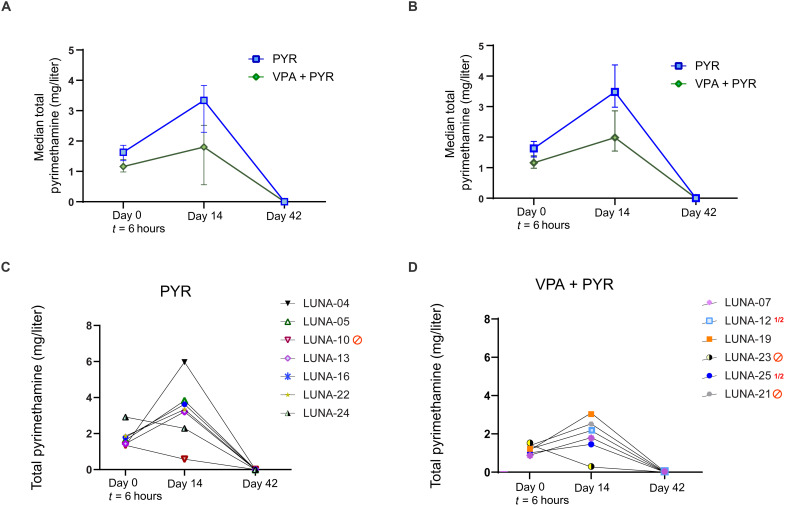
Fig. 4. Pharmacokinetics of the drug pyrimethamine in study participants.
(A and B) Pharmacokinetic analysis of the drug pyrimethamine in study participants from pyrimethamine (blue) and the combination treatment arm with pyrimethamine and VPA (green) as measured by median total pyrimethamine (mg/liter) levels in plasma with IQRs at 6 hours after first dosing (day 0, t = 6 hours), at the end of treatment period (day 14), and 28 days after the end of treatment (day 42) overall (A) and in those with uninterrupted pyrimethamine exposure (B) and represented as plasma pyrimethamine levels at the time points per individual on pyrimethamine (C) or the combination treatment with pyrimethamine and VPA (D).