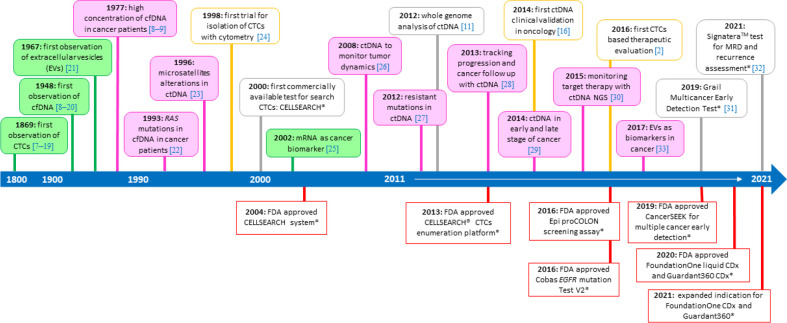
Figure 1.
The timeline of liquid biopsy development. The figure illustrates some of the significant events in the development and spread of the liquid biopsy in clinical practice. * Some of the Food and Drug Administration (FDA) approved tests, addressed in the following text of the manuscript (“ctDNA liquid biopsy assays” and “Clinical applications of ctDNA liquid biopsy”); NGS: next generation sequencing; MRD: minimal residual disease