Figure 5.
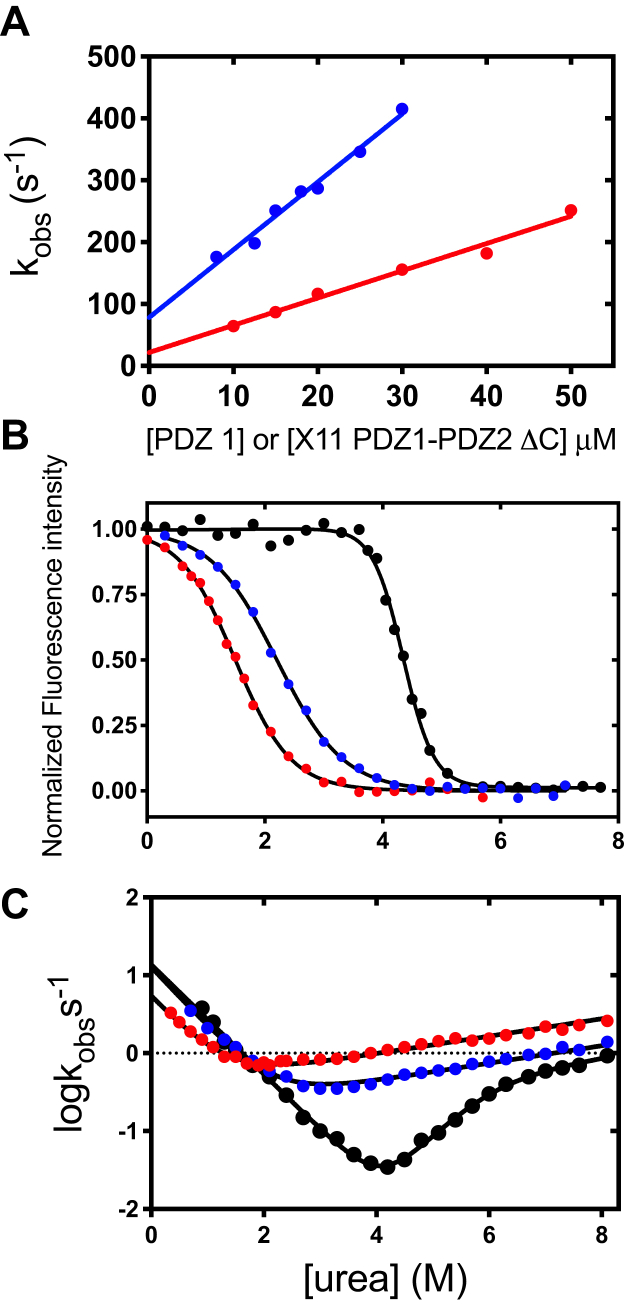
Folding and binding properties of X11 PDZ1-PDZ2 ΔC.A, pseudo-first-order binding plots for PDZ1 (red) and X11 PDZ1-PDZ2 ΔC (blue). No binding could be observed in the case of X11 PDZ1-PDZ2, further confirming the autoinhibitory role of the C-terminal tail. B, fluorescence monitored equilibrium denaturation of X11 PDZ1-PDZ2 ΔC (blue) in comparison to X11 PDZ1-PDZ2 (black) and PDZ1 (red). Lines are the best fit to a two-state transition. It is evident that truncation of solely nine amino acids, in the case of X11 PDZ1-PDZ2 ΔC, causes a remarkable decrease in cooperativity that is similar to PDZ1 in isolation. C, chevron plot of X11 PDZ1-PDZ2 ΔC (blue), in comparison with X11 PDZ1-PDZ2 (black) and PDZ1 (red). It is evident that deletion of solely nine amino acids decreases the apparent cooperativity with folding kinetics of PDZ1-PDZ2 ΔC closely resembling that observed for PDZ1.