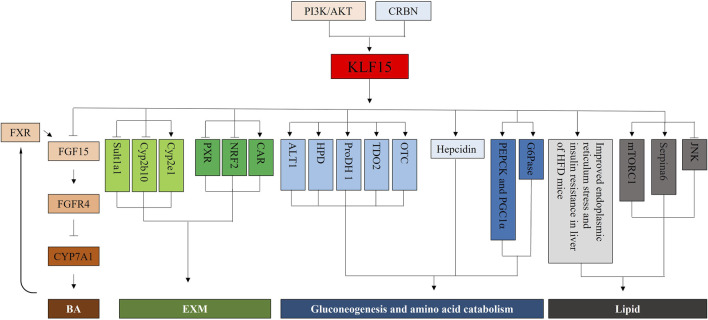
FIGURE 4.
Mechanistic illustrations. First, KLF15, a direct negative regulator of ileal FGF15 and FGFR4, is critical for Cyp7a1 expression and BA synthesis in the liver. The BA synthesis is also mediated by PI3K/AKT/KLF15 signaling. Second, KLF15 represses phase I–II targets (Sult1a1, Cyp2b10) but induces Cyp2e1. And, KLF15 deficiency modestly enhances PXR and NRF2 expression, but decreases CAR. Third, KLF15−/− mice manifests a decrease in the expression of genes for enzymes that mediate amino acid degradation, including those for ALT1, HPDProDH, TDO2, and OTC. Acute depletion of KLF15 by RNAi inhibits the expression of gluconeogenic or amino acid-degrading enzymes, such as PEPCK, PGC1α and G6Pase, in cultured hepatocytes. Moreover, CRBN and KLF15 are mediators of fasting-induced hepatic hepcidin expression and its biosynthesis. Finally, Deletion of the KLF15 gene improves endoplasmic reticulum stress and insulin resistance in liver of HFD mice. Inhibition of the KLF15 gene promotes the expression of JNK phosphorylation, and inhibits the mTORC1 signaling pathway. Hepatocyte KLF15 regulates plasma corticosteroid transport and thereby inflammatory homeostasis via direct and specific transcriptional activation of Serpina6.