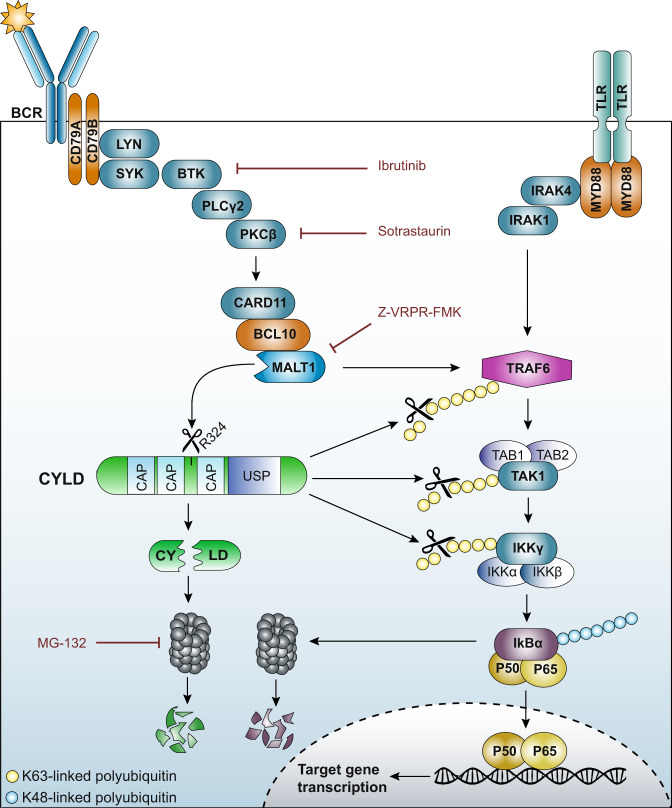
Fig. 7. Model of the role of CYLD in NF-κB activation in B-cell lymphomas.
Upon B-cell receptor (BCR) ligation, tyrosine residues within the ITAM motifs of CD79 are phosphorylated by the Src-family tyrosine kinase LYN leading to activation of spleen tyrosine kinase (SYK). Subsequently, Bruton’s tyrosine kinase (BTK) is activated and can then phosphorylate phospholipase Cγ2 (PLCγ2). PLCγ2 mediates the formation of second messengers that activate protein kinase Cβ (PKCβ). PKCβ phosphorylates caspase recruitment domain-containing protein 11 (CARD11) provoking a conformational change and allowing CARD11 to interact with B-cell lymphoma 10 (BCL10), and subsequently MALT1. MALT1 is a protease that cleaves various target proteins, including CYLD. In addition, oligomerized MALT1 functions as a scaffolding protein allowing recruitment of the E3 ubiquitin ligase tumor necrosis factor receptor-associated factor 6 (TRAF6). In parallel, Toll-like receptor (TLR) engagement results in MyD88-dependent recruitment of IL-1 receptor-associated kinase-4 (IRAK4) and subsequently IRAK1. IRAK4 phosphorylates IRAK1, which then can associate with TRAF6. BCR/TLR-activated TRAF6 promotes Lys-63-linked ubiquitination of TRAF6 itself as well as transforming growth factor beta-activated kinase 1 (TAK1) and NEMO/IKK-γ. Ubiquitinated TRAF6 binds to adaptor proteins TAB1/2/3, leading to the recruitment and auto-phosphorylation of TAK1. Ubiquitination of NEMO/IKK-γ mediates the recruitment of the IKK subunits to the TAK1/TAB complex, thereby facilitating the phosphorylation of IKK-β by TAK1. IKK-β then phosphorylates IκBα resulting in Lys-48-polyubiquitination and subsequent proteasomal degradation which allows NF-κB dimers to translocate to the nucleus. The deubiquinating enzyme CYLD consists of three conserved cytoskeleton-associated protein glycine-rich (CAP-Gly) domains and a C-terminal catalytic ubiquitin-specific protease (USP) domain that is able to hydrolyze lysine 63-linked ubiquitin chains. CYLD can hydrolyze Lys-63-linked polyubiquitin chains of TRAF6, TAK1 and/or NEMO/IKK-γ, thereby suppressing NF-κB activation. Accordingly, MALT1-dependent cleavage of CYLD substantially reduces its functionality and initiates its proteasomal degradation, thereby promoting cell growth and NF-κB activation.