Abstract
Background Obstructive sleep apnea (OSA) is associated with many diseases, but evidence indicating that OSA is a risk factor for dyslipidemia is lacking. Aim This cross-sectional study investigated the prevalence of lipid abnormalities in patients with OSA and its association with OSA severity. Material and Methods: In this cross-sectional study, 102 patients with suspected OSA underwent standard polysomnography. All patients with an apnea-hypopnea index (AHI) of ≥5 with symptoms were diagnosed as having OSA. A fasting blood sample was collected from all patients. Blood levels of triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL) were measured. The relationship between the AHI and lipid profiles was analyzed, and linear regression analysis was performed to evaluate the effect of dyslipidemia on OSA. Results: The patients with OSA had a significantly higher TG level and a significantly lower HDL level than did those without OSA. The lipid abnormalities increased with OSA severity. The mean serum TG level was higher in the severe OSA group (175±46.5 vs. 153±42.45, mg/dl P = 0.048), and the mean serum HDL level was lower in the severe OSA group (38.43 ± 5.19 vs. 45.73 ± 4.98, mg/dl P = 0.004). Serum TG, cholesterol, and LDL levels were correlated with a BMI of <30 and a BMI of >30 in the OSA group. Linear regression analysis indicated that only age (β = 0.301, P = 0.000), BMI (β = 0.455, P = 0.000), serum HDL level (β = -0.297, P = 0.012), and serum LDL level (β = 0.429, P = 0.001) were the independent predictors of OSA. Conclusion: OSA and obesity are potential risk factors for dyslipidemia. The diagnosis of hyperlipidemia was linked to OSA, and the association was more significant with OSA severity. Hyperlipidemia was well recognized in patients with OSA. LDL and HDL are the independent predictors of OSA.
Keywords: Polysomnography, obesity, risk factor, raised LDL
Introduction
Obstructive sleep apnea (OSA) is a prevalent sleep disorder affecting up to 20% of the general population and approximately 4% of men and 2% of women in their middle years [1]. OSA is a sleep disease characterized by recurrent upper airway obstruction that may cause arousal, sleep fragmentation, intermittent hypoxia (IH), and excessive daytime sleepiness [2]. The most common symptoms of OSA are sleepiness, fatigue, nonrestorative sleep, chronic snoring, choking, and breathing interruption during sleep.
OSA is associated with decreased alertness, productivity, and workplace or vehicular accidents. OSA substantially increases the likelihood of being involved in a car accident. Treatment for OSA, such as continuous positive airway pressure (CPAP) therapy, can significantly reduce this risk [3]. Screening tools, such as the Berlin Questionnaire, STOP-BANG Questionnaire, and Epworth Sleepiness Scale, can be used to identify patients with a risk of OSA. Polysomnography (PSG) is the gold standard for diagnosing OSA. Patients with moderate and severe OSA should receive treatment. The American Academy of Sleep Medicine guidelines recommend positive airway pressure (PAP) as the treatment of choice for all severities of OSA [4]. Evidence indicating that OSA causes metabolic problems and is a risk factor for dyslipidemia is lacking. Changes in essential physiological functions, such as an increase in the heart rate, sympathetic activity, oxidative stress, insulin resistance, and endothelial dysfunction, have been linked to OSA [5].
OSA and dyslipidemia might be causally associated. Oxidative stress, insulin resistance, and endothelial dysfunction have been related to a causal relationship between OSA and metabolic syndrome [6].
In patients with OSA, mechanical obstruction of the airway results in IH. IH can cause alterations in lipid metabolism. Dyslipidemia caused by IH contributes to the development and progression of atherosclerosis [7]. Hypoxia may disturb the balance between lipid storage and mobilization in hepatic and adipose tissues. These disturbances, characterized by the overproduction and impaired clearance of triglyceride (TG)-rich lipoproteins, can lead to the deterioration of the blood lipid profile, specifically an increase in the plasma TG level [8]. Several cross-sectional studies suggest that OSA is independently associated with increased total cholesterol (TC), low-density lipoprotein (LDL), and TG levels. In addition, a study reported that CPAP treatment may have a beneficial effect on the lipid profile in patients with OSA [9]. An animal studies demonstrated that IH is a direct cause of hyperlipidemia. The magnitude of hyperlipidemia was correlated with the extent of the hypoxia stimulus [10].
Another crucial risk factor for atherosclerosis and cardiovascular disease is dyslipidemia, which is characterized by elevated blood TC and LDL-cholesterol (LDL-C) levels (ASCVD). Moreover, dyslipidemia is defined as increases in TC (≥200 mg/dL), LDL (≥100 mg/dL), and TGs (≥150 mg/dL) and a reduction in the high-density lipoprotein cholesterol (HDL; ≤40 mg/dL in men and ≤45 mg/dL in women) [11]. Many epidemiological and interventional studies have consistently demonstrated a linear relationship between serum LDL-C levels and incident ASCVD [12]. This study examined the association between the prevalence of dyslipidemia and the severity of OSA.
Material and methods
Study type
This study examined 119 patients with OSA over a period of 1 year by using the STOP-BANG Questionnaire. Finally, in this prospective observational study, 102 patients were included after the exclusion of 17 patients (Figure 1). This study was conducted at the Department of Respiratory Medicine, King George’s Medical University, Lucknow.
Figure 1.
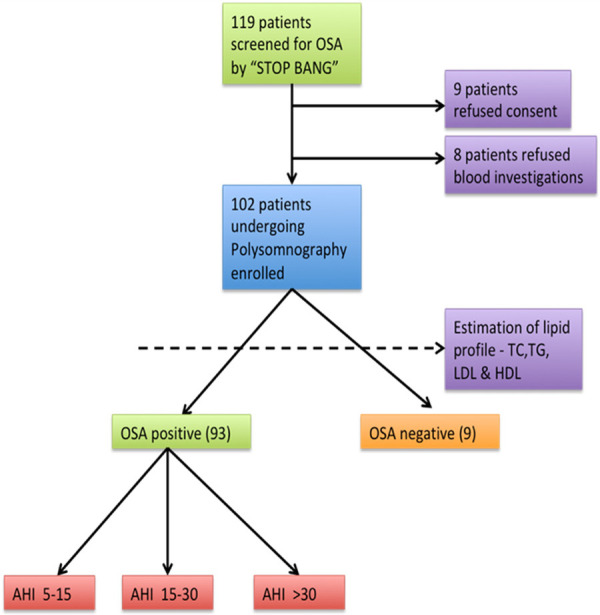
Study flow chart.
Inclusion criteria
Patients who were aged from 25 to 60 years and met at least 3 of the 8 STOP-BANG criteria for OSA were included in this study. The criteria were as follows: snoring, tiredness, observed apnea, blood pressure >140/90 mmHg, body mass index >30 kg/m2, age >50 years, and neck circumference >17 inches for men and >16 inches for women. The most common symptoms were snoring, observed apnea, and daytime sleepiness.
Exclusion criteria
Patients who were previously diagnosed as having diabetes, lipid abnormalities, chronic kidney disease, chronic liver disease, chronic pulmonary disease, and chronic heart disease were excluded from this study. In addition, patients receiving lipid-lowering drugs and oral steroids were excluded.
Ethics statement
This study was approved by the institutional ethics committee Reference code 98th ECM IIB thesis/P8. All patients provided informed consent. The polysomnographic scan was conducted to determine the diagnosis of obstructive sleep apnea and determine its severity. Level 1 polysomnography was used to calculate the Apnea-Hypopnea Index (AHI), which is the number of apneas or hypopneas recorded during the study per hour of sleep (PSG).
Hypopneas were defined as a drop of 50% or more in nasal flow and associated with 3% or more oxygen desaturation. Standard overnight PSG (Nicolet UltraSom System, Madison, WI, USA) was performed in all the patients in a sleep laboratory to determine sleep parameters and architecture. All PSG results were scored manually in accordance with standard criteria [13]. All the patients with an apnea-hypopnea index (AHI) of >5 with symptoms were diagnosed as having OSA. The patients with OSA were divided into three groups on the basis of AHI criterion: mild OSA (AHI score of 5-14.9), moderate AHI (AHI score of 15-29.9), and severe OSA (AHI score of >30). We examined two groups: those with mild to moderate OSA and those with severe OSA. A blood sample (preferably after 12 hours of overnight fasting) was collected from all the patients. The blood levels of TGs, TC, HDL, and LDL were measured. Friedwald’s formula was used to estimate the serum LDL level except when the TG level was >350 mg % [14]. Anthropometric and demographic data in terms of age, sex, BMI (Body mass index) were also analysed. Corelation of various lipid parameters with BMI were analyzed.
Statistical analyses
Data are expressed as the mean and standard deviation (SD). Comparisons between the groups with and without OSAS were performed using the unpaired t test for parametric variables. The relationship between the AHI and lipid profiles was analyzed, and linear regression analysis was performed to evaluate the effect of OSA on dyslipidemia. All statistical analyses were performed using Statistical software (Stat Soft version 17, Santa Clara, USA). A p value of <0.05 was considered statistically significant.
Results
Baseline characteristics
The study population (n = 102) was divided into the OSA (n = 93) and non-OSA (n = 9) groups on the basis of PSG findings. The patients with OSA were classified into mild (AHI = 5-14.9 events/h, n = 3), moderate (AHI = 15-29.9 events/h, n = 16), and severe (AHI >30 events/h, n = 74) subgroups (Figure 2). The patients with OSA were significantly older, had higher BMI, and were predominantly men (all P<0.05; Table 1) than did those without OSA. Of the 93 patients with OSA, 58 (62.36%) were men, with a mean age of 49.91±8.4 years. A total of 37 (36.3%) patients were smokers in the OSA group. The mean STOP-BANG score was 6, whereas snoring was noted in 82 (80.4%) patients. The mean AHI of the OSA group was 48.43±21.8.
Figure 2.
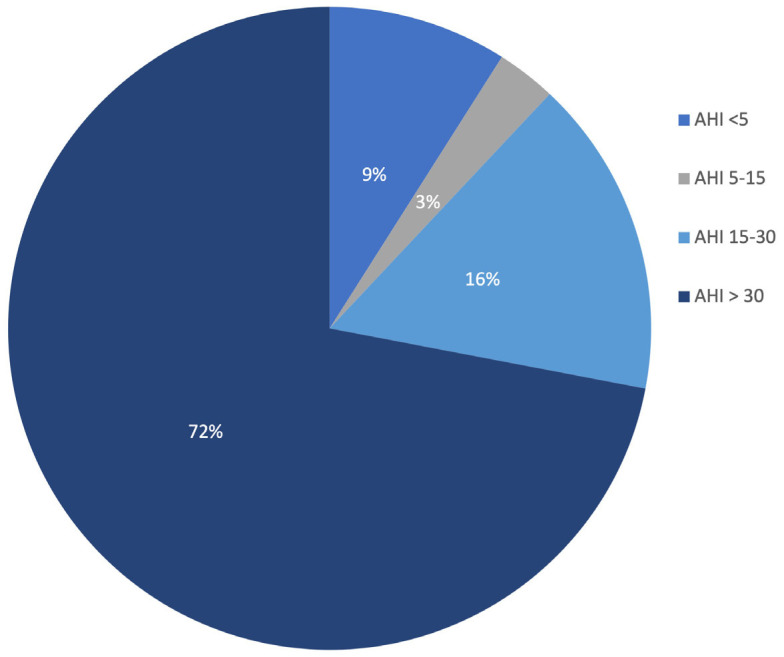
Distribution of AHI*. *AHI-Apnoea Hypopnea Index.
Table 1.
Baseline characteristics of the study population
| OSA Positive * (n = 93) | OSA Negative * (n = 9) | P ** value | |
|---|---|---|---|
| Age | 50.45±8.4 years | 44.33±6.5 | .037 |
| Male | 58 (62.3%) | 6 (66.6%) | |
| M:F ratio | 1.31:1 | 2:1 | |
| Smoker | 37 (39.7%) | 2 (22.2%) | |
| BMI | 29.08±4.27 | 24.15±5.02 | .04 |
| STOP-BANG score | 6 | 3 | |
| Tobacco Chewer | 20 (21.5%) | 1 (11.1) | |
| Snoring | 82 (80.4%) | 3 (33.3%) | |
| AHI | 48.43±21.8 | 4.22±3.86 | .003 |
| S.T.G | 177.46±45.38 | 151.33±46.53 | .04 |
| S.LDL | 105.83±36.01 | 96.11±22.95 | |
| S.TC | 210.40±62.94 | 191.67±45.02 | |
| S.HDL | 39.78±5.19 | 45.12±11.9 | .022 |
OSA-Obstructive Sleep Apnea.
P < 0.05 is significant.
BMI and OSA
The mean BMI of the study population was 29.08±4.27. According to BMI, 61 (59.80%) patients were determined to not have obesity (BMI ≤30 kg/m2) and 41 (40.19%) were determined as having obesity (BMI >30 kg/m2). In the non obese group (BMI <30), 6 (6.45%) patients were not diagnosed as having OSA and 55 (59.13%) were diagnosed as having OSA. In the obese group, 3 (3.22%) patients were not diagnosed as having OSA and 38 (40.86%) were diagnosed as having OSA. OSA was prevalent in both the groups irrespective of BMI (Figure 3).
Figure 3.
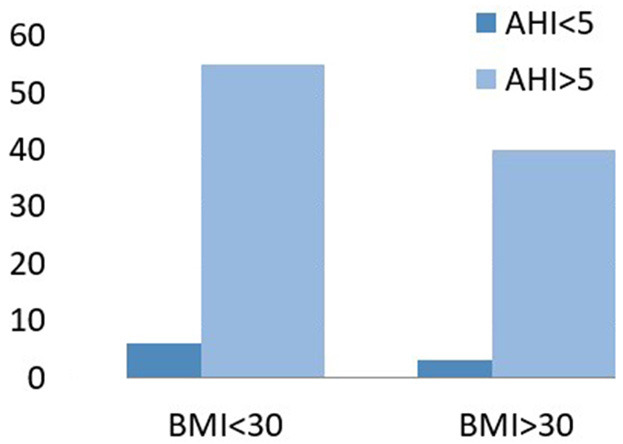
OSA is prevalent irrespective of BMI.
Lipid profile and OSA
In the OSA group, 69.8% (n = 65) of patients had dyslipidmeia. Out of these patients, 51.6% (n = 48), 25.8% (n = 24), 69.8% (n = 65), and 43 (46.2%) had hypercholesteremia, higher serum LDL levels, higher serum TG levels, and lower serum HDL levels respectively. The mean serum TG, TC, LDL, and HDL levels were 177.46±45.38, 210.40±62.94, 105.83±36.01, and 39.78±5.19, respectively, in the OSA group.
Lipid profile and severity of OSA
The prevalence of dyslipidemia increased with the AHI. In addition, the TG, TC, and LDL levels increased with AHI (Figures 4 and 5). The mean lipid levels were higher in the patients with severe OSA than in those with mild to moderate OSA (Table 2). Only serum TG and HDL levels significantly differed between the two groups. However, a significant association was noted between AHI and TG levels (P = 0.048) and between AHI and HDL levels (P = 0.004) but not with other lipid components (Table 2).
Figure 4.
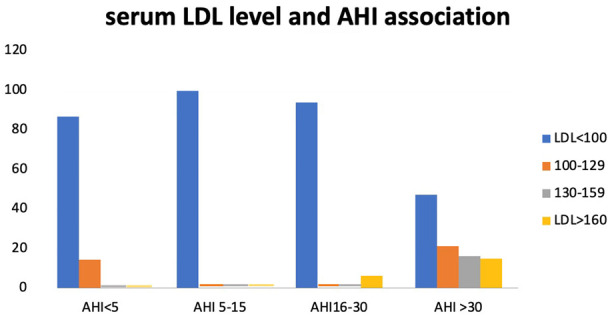
Serum LDL level in different AHI groups. Chi-square-X2 = 22.6; P = 0.007.
Figure 5.
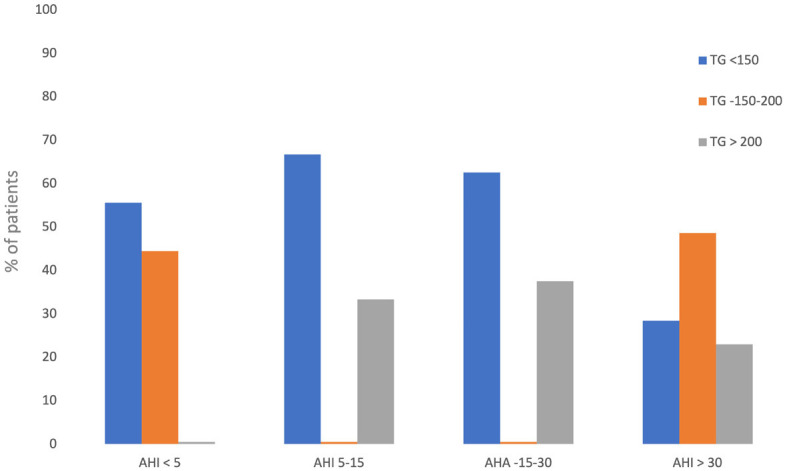
Serum TG levels in different AHI groups. Chi-square-X2 = 18.575; P = 0.005.
Table 2.
Lipid parameters in mild-moderate and severe OSA group
| AHI 5-29.9 | AHI >30 | P value | |
|---|---|---|---|
| TG | 153±42.45 | 175±46.5 | .048 |
| TC | 190±45.64 | 204.75±48.65 | NS |
| HDL | 45.73±4.98 | 38.43±5.19 | .004 |
| LDL | 110±41.32 | 124.87±43.45 | NS |
AHI-Apnoea Hypopnea index, TG-Triglyceride, TC-Total Cholesterol, HDL-High Density Lipoprotein, LDL-Low Density Lipoprotein.
Hypertriglyceridemia was observed in 4 (44.4%) patients in the non-OSA group, in moderate OSA group 36.84%, and in severe OSA group 71.6% (Figure 5).
The mean serum TG and TC levels were higher in the patients with a BMI of ≥30 than in those with a BMI of <30, whereas the mean serum LDL level was higher in the patients with a BMI of <30 (Table 3). The mean serum TG and HDL levels significantly differed between the patients with a BMI of ≥30 and those with a BMI of <30 (independent samples t test; P<0.05). A linear regression analysis was performed to predict the AHI based on different lipid levels, sex, age, and BMI. When all the parameters were analyzed with linear regression, only age (β = 0.301, P = 0.000), BMI (β = 0.455, P = 0.000), and LDL levels (β = 0.429, P = 0.001) were determined as the independent predictors of OSA. A scatter plot revealed the correlations of serum TG, LDL, and TC levels with the AHI (Figure 6).
Table 3.
Lipid parameters in obese vs. non obese OSA group
| BMI | N | Mean ± Std. Deviation | |
|---|---|---|---|
| S.TG | ≥30.00 | 41 | 192.62±31.15 |
| <30.00 | 61 | 166.31±50.57 | |
| S.LDL | ≥30.00 | 41 | 102.70±31.74 |
| <30.00 | 61 | 107.034±38.01 | |
| S.HDL | ≥30.00 | 41 | 48.01±11.57 |
| <30.00 | 61 | 48.21±11.58 | |
| Total Cholesterol | ≥30.00 | 41 | 211.70±60.05 |
| <30.00 | 61 | 207.03±64.35 |
Figure 6.
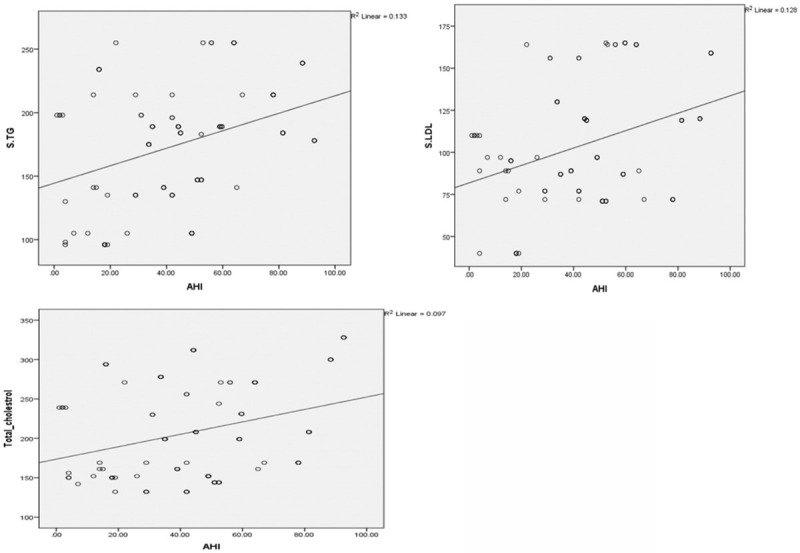
A scatter plot showing correlation of serum TG, Serum LDL, Serum cholesterol with AHI.
Discussion
In this prospective observational study of 93 newely diagnosed obstructive sleep aponea patients we showed a significant correlation of lipid abnormalities and Apnea-Hypopnea Index (AHI) - an indicator of obstructive sleep apnea severity.
Studies have recently suggested the involvement of OSA in the development of metabolic syndrome, including dyslipidemia, a surrogate marker for atherosclerosis. OSA may be associated with metabolic abnormalities. Mechanisms through which OSA can disrupt metabolism are complex. Many pathogenic mediating pathways (sympathetic activation, neurohumoral changes, glucose homeostasis disturbance, inflammation, and oxidative stress) may activate chronic IH, resulting in the deterioration of metabolic function [15,16].
Link between AHI and lipid: The present study investigated the link between obstructive respiratory events (AHI) and the lipid profile. Triglycerides, total cholesterol, and LDL were lipid abnormalities connected to the severity of OSA in our study. There were more significant lipid changes in patients with severe obstructive sleep apnea (AHI > 30).
Our results revealed that the patients with OSA had significantly higher serum TG, TC, and LDL levels and a lower serum HDL-cholesterol level. The AHI was inversely associated with the HDL cholesterol level in young men and the TG level in women alone in the American Heart Health Sleep Study [16]. By contrast, Lam et al examined 255 patients aged between 30 and 60 years and observed no correlation between OSA and the HDL or TG level [17].
In our study with the increase in OSA severity, the lipid parameters worsened; a higher AHI value was associated with higher TG, TC, and LDL levels.
Our study demonstrated that the patients with OSA had a higher BMI, were older, and were predominantly men. A similar study reported a 2.64-fold increase in the prevalence of OSA in men [18]. This result is compatible with the literature finding of OSA being more prevalent in men. The mean age of our study population was 50.45±8.4 years, which is similar to that reported in previous studies [7,19].
Prevelance of dyslipidemia: Chou et al noted a high prevalence of hypercholesterolemia and hypertriglyceridemia at 61.1% and 55.3%, respectively, similar to our database [7]. Another study reported that the prevalence of hypercholesterolemia was 47% and an increased LDL was noted in 38% patients. Hypertriglyceridemia was less prevalent, affecting approximately 13% of the population [19]. A cross-sectional study reported that the prevalence of hyperlipidemia increased from 15.1% in the patients without OSA to 26.1% in the patients with severe OSA [20].
The overall prevalence of dyslipidemia in the OSA group was 69.8% in our study. A study reported that the majority of Indian patients exhibit lipid derangement [21].
OSA and BMI: In our study 59.13% patients were diagnosed with OSA in non obese group (BMI <30) and 40.86% patients were diagnosed with OSA in obese group (BMI ≥30). In our study we observed no significant difference in the prevalance of OSA with BMI.
The mean, TG, levels were significantly higher in patients with OSA with BMI >30 than OSA patients with BMI <30. The mean HDL levels were higher in lower BMI with OSA patients [22]. Total cholesterol, LDL levels were higher in OSA with obese patients but it was non significant. Studies investigating the relationship between OSA and dyslipidemia have indicated a role of obesity. Although some studies have reported no correlation between OSA and hyperlipidemia outside of the effect of obesity, some have indicated that OSA and hyperlipidemia are linked in a manner that is independent of BMI.
In our study, dyslipidemia is a comorbidity related to OSA, studies on such association are scant, and clinical evidence is limited. Some studies have indicated an existing relationship between the increased prevalence of dyslipidemia and OSA, but a clear association has not yet been identified [23-25].
Dyslipidemia and OSA severity
Dyslipidemia and OSA severity could be related, with higher levels of dyslipidemia being associated with an increased AHI as similar to our study [26]. A meta-analysis reported that the AHI exerted a significant effect on HDL and TG only [27]. In our study, we also found signifcant relation of AHI with low HDL & high TG but not with other lipid parameters. Although a clear causal relationship of OSA with dyslipidemia is yet to be demonstrated, chronic IH, a significant component of OSA, is independently associated with dyslipidemia and might cause dyslipidemia through the generation of stearoyl coenzyme A debate-rase-1 and reactive oxygen species, peroxidation of lipids, and the dysfunction of the sympathetic system [28].
The effect of OSA on hyperlipidemia was examined on the basis of lipid concentrations instead of hyperlipidemia diagnosis. Only three studies identified a significant relationship between OSA severity and lipid concentrations in a meta-analysis of 13 cross-sectional studies. Moreover, several studies reported a weak or no association between the two variables [28].
The European Sleep Apnea database cohort discovered a robust linear relationship between OSA severity and various lipid concentrations (TC, HDL- and LDL-cholesterol, and TGs) in patients with OSA who did not have a documented diagnosis of hyperlipidemia or received lipid-lowering medications [14]. These findings are congruent with those of our study; we determined that despite the creation of a separate risk of hyperlipidemia, morbid obesity had no effect on the link between OSA and hyperlipidemia. The independent relationship between the measures of OSA (AHI) and the lipid profile (TC, HDL-C, LDL-C, and fasting TGs) was investigated using a general linear model. Dyslipidemia is a known risk factor for cardiovascular disease.
This study including patients with newly diagnosed OSA observed a strong correlation between lipid abnormalities and OSA severity. In our study, the severity of OSA was associated with blood TG and HDL levels. Enhanced hepatic synthesis of TGs and cholesterol, slower clearance due to lipase activity suppression, and increased mobilization of free fatty acids from the adipose tissue are key factors contributing to elevated cholesterol and TG levels in individuals with OSA [29]. Our study observed a strong relationship between HDL levels and OSA severity, suggesting that the patients with a lower HDL level have a higher AHI. HDL exerts a protective effect on the cardiovascular system; however, some HDL subfractions, particularly small HDL subfractions such as small LDL3-7 and small HDL8-10, exert atherogenic effects on patients with OSA. The two main parameters affecting the antioxidant activity of HDL are higher HDL lipid peroxide levels and lower serum paraoxonase-1 activity. Moreover, larger HDL subfractions may exhibit poorer antioxidant activity. This may be the reason why people with OSA who have normal HDL levels do not consistently demonstrate the preventive effects of HDL. Patients with decreased antioxidant HDL activity also exhibited elevated levels of intercellular adhesion molecule 1 and tumor necrosis factor [30]. The age, BMI and LDL levels were determined as the independent predictors of OSA in our study.
Conclusion
The diagnosis of hyperlipidemia is linked to OSA, and the association is more significant for TG & HDL. Hyperlipidemia was noticeably underrecognized in patients with OSA.
Limitation
This is a single-center study including a small sample size. No follow-up was conducted for patients with OSA. The study did not reveal the effect of therapy on the lipid profile.
Disclosure of conflict of interest
None.
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karimi M, Hedner J, Häbel H, Nerman O, Grote L. Sleep apnea-related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish traffic accident registry data. Sleep. 2015;38:341–9. doi: 10.5665/sleep.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2019;15:335–343. doi: 10.5664/jcsm.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almendros I, García-Río F. Sleep apnoea, insulin resistance and diabetes: the first step is in the fat. Eur Respir J. 2017;49:1700179. doi: 10.1183/13993003.00179-2017. [DOI] [PubMed] [Google Scholar]
- 6.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM Jr, Ridker PM, Grundy SM, Kastelein JJ. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–94. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou YT, Chuang LP, Li HY, Fu JY, Lin SW, Yang CT, Chen NH. Hyperlipidaemia in patients with sleep-related breathing disorders: prevalence & risk factors. Indian J Med Res. 2010;131:121–5. [PubMed] [Google Scholar]
- 8.Gangwar A, Paul S, Ahmad Y, Bhargawa K. Intermittent hypoxia modulates redox homeostasis, lipid metabolism associated inflammatory processes and redox post-translational modifications: benefits at high altitude. Sci Rep. 2020;10:7899. doi: 10.1038/s41598-020-64848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokuda F, Sando Y, Matsui H, Koike H, Yokoyama T. Serum levels of adipocytokines, adiponectin and leptin, in patients with obstructive sleep apnea syndrome. Intern Med. 2008;47:1843–9. doi: 10.2169/internalmedicine.47.1035. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol. 2007;102:557–63. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- 11.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59:777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM. American academy of sleep medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.McNicholas WT, Bonsigore MR Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 15.Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–260. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 16.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the sleep heart health study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 17.Lam JC, Lam B, Lam CL, Fong D, Wang JK, Tse HF, Lam KS, Ip MS. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respir Med. 2006;100:980–7. doi: 10.1016/j.rmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–14. [PubMed] [Google Scholar]
- 19.Adedayo AM, Olafiranye O, Smith D, Hill A, Zizi F, Brown C, Jean-Louis G. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath. 2014;18:13–8. doi: 10.1007/s11325-012-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunduz C, Basoglu OK, Hedner J, Bonsignore MR, Hein H, Staats R, Bouloukaki I, Roisman G, Pataka A, Sliwinski P, Ludka O, Pepin JL, Grote L European Sleep Apnoea Database collaborators. Hyperlipidaemia prevalence and cholesterol control in obstructive sleep apnoea: data from the European sleep apnea database (ESADA) J Intern Med. 2019;286:676–688. doi: 10.1111/joim.12952. [DOI] [PubMed] [Google Scholar]
- 21.Hussain A, Ali I, Kaleem WA, Yasmeen F. Correlation between body mass index and lipid profile in patients with type 2 diabetes attending a tertiary care hospital in Peshawar. Pak J Med Sci. 2019;35:591–597. doi: 10.12669/pjms.35.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30:29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallmeier D, Koenig W. Strategies for vascular disease prevention: the role of lipids and related markers including apolipoproteins, low-density lipoproteins (LDL)-particle size, high sensitivity C-reactive protein (hs-CRP), lipoprotein-associated phospholipase A2 (Lp-PLA2) and lipoprotein(a) (Lp(a)) Best Pract Res Clin Endocrinol Metab. 2014;28:281–94. doi: 10.1016/j.beem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Gasa M, Salord N, Fortuna AM, Mayos M, Vilarrasa N, Dorca J, Montserrat JM, Bonsignore MR, Monasterio C. Obstructive sleep apnoea and metabolic impairment in severe obesity. Eur Respir J. 2011;38:1089–97. doi: 10.1183/09031936.00198810. [DOI] [PubMed] [Google Scholar]
- 26.Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, Kramer MR. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med. 2007;101:1696–701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, Naseem J, Champeau D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10:475–89. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gündüz C, Basoglu OK, Hedner J, Zou D, Bonsignore MR, Hein H, Staats R, Pataka A, Barbe F, Sliwinski P, Kent BD, Pepin JL, Grote L. European sleep apnea database collaborators. Obstructive sleep apnoea independently predicts lipid levels: data from the European Sleep Apnea Database. Respirology. 2018;23:1180–1189. doi: 10.1111/resp.13372. [DOI] [PubMed] [Google Scholar]
- 29.Kollar B, Siarnik P, Hluchanova A, Klobucnikova K, Mucska I, Turcani P, Paduchova Z, Katrencikova B, Janubova M, Konarikova K, Argalasova L, Oravec S, Zitnanova I. The impact of sleep apnea syndrome on the altered lipid metabolism and the redox balance. Lipids Health Dis. 2021;20:175. doi: 10.1186/s12944-021-01604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inonu Koseoglu H, Pazarli AC, Kanbay A, Demir O. Monocyte count/HDL cholesterol ratio and cardiovascular disease in patients with obstructive sleep apnea syndrome: a multicenter study. Clin Appl Thromb Hemost. 2018;24:139–144. doi: 10.1177/1076029616677803. [DOI] [PMC free article] [PubMed] [Google Scholar]


