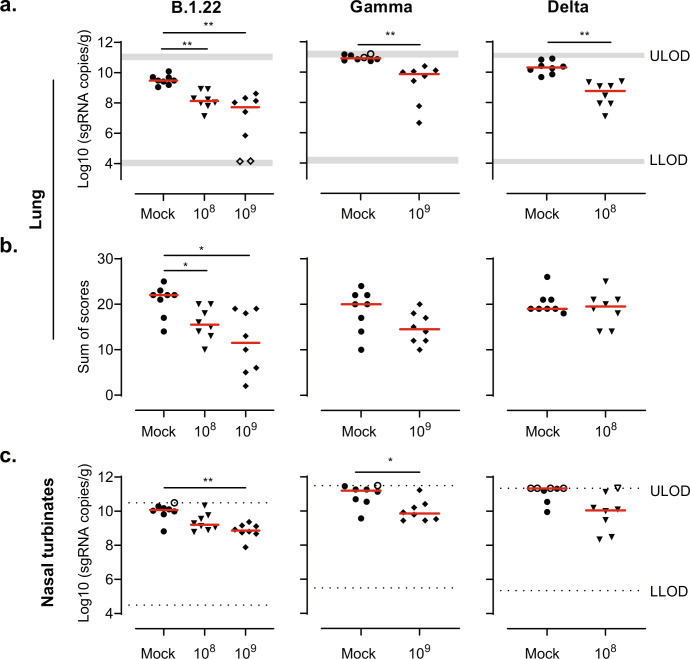
Fig. 2. Ad26.COV2.S reduces the viral load and lung histopathology after challenge with SARS-CoV-2 B.1.22, Gamma and Delta.
Hamsters were vaccinated with formulation buffer (mock), 108 or 109 vp Ad26.COV2.S at day −28 (n = 8 per group). The animals were intranasally challenged with 103 TCID50 SARS-CoV-2 B.1.22, 104 TCID50 SARS-CoV-2 Gamma, or 104 TCID50 SARS-CoV-2 Delta on day 0. While animals vaccinated with 108 and 109 vp were evaluated on day 4 after the challenge with SARS-CoV-2 B.1.22, only animals vaccinated with 109 vp were examined on day 4 after the challenge with SARS-CoV-2 Gamma, and only 108 vp-vaccinated animals were evaluated at day 4 after challenge with the Delta variant. SARS-CoV-2 Envelope subgenomic RNA (sgRNA) was measured in the a lungs and c nasal turbinates (nose) on day 4. b Paraffin sections from lung tissue (H&E) were scored at day 4 for alveolar edema, hemorrhage, infiltrate, alveolar and/or interstitial inflammation, bronchitis and/or bronchiolitis, hyperplasia and/or hypertrophy mucous cells, peribronchiolar and/or perivascular cuffing, pleural fibrosis, thickening of alveolar septa and type II pneumocyte hyperplasia. The sum of scores is presented (potential range 0–48). Red horizontal bars indicate the median response per group and the dotted line/gray zone indicates the limit of detection (LOD) or LOD range. Open symbols indicate the response is at or below the lower LOD (LLOD)/at or above the upper LOD (ULOD). Comparisons were performed by a Mann–Whitney U test with a twofold Bonferroni correction. Statistical differences are indicated by asterisks: *P < 0.05, **P < 0.01.