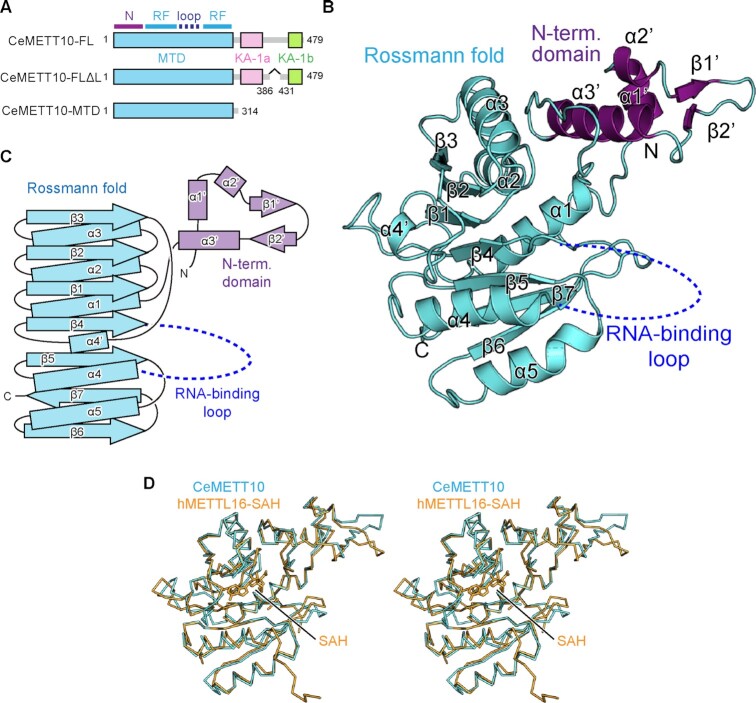
Figure 1.
Overall structure of C. elegans METT10-MTD. (A) Schematic diagrams of full-length C. elegans METT10 (CeMETT10-FL), its variant CeMETT10-FLΔL lacking the putative disordered region (residues 386–430), and its N-terminal methyltransferase domain (CeMETT10-MTD: residues 1–314). The MTD is colored cyan, and the C-terminal kinase-associated domains, KA-1a and KA-1b, are magenta and green, respectively. The region between KA-1a and KA-1b is predicted to be disordered. The MTD consists of the N-terminal domain (N) and Rossmann fold (RF). (B) Overall structure of CeMETT10-MTD. Residues 6–187 and 236–309 are modeled in the structure. The extended N-terminal domain and the Rossmann fold are colored magenta and cyan, respectively, and the RNA binding loop (dashed line) between β4 and α4 was disordered and not modeled in the structure. (C) Schematic view of the secondary structure of CeMETT10-MTD. The N-terminal domain and the Rossmann fold are colored as in (B). (D) Stereo view of the structural alignment of Cα atoms between CeMETT10-MTD (cyan) and human METTL16-MTD (hMETTL16-MTD) in complex with SAH (orange, PDB: 6B92) (34).