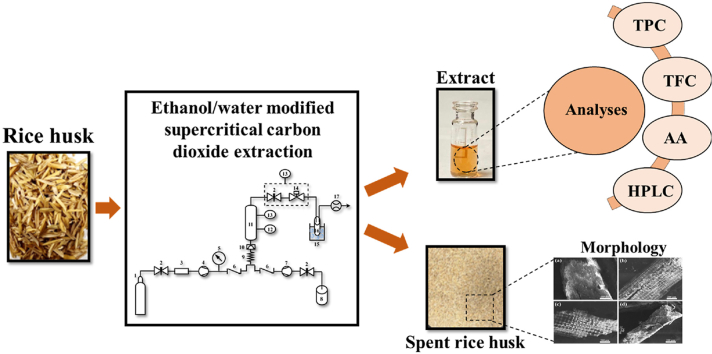
Abstract
Rice husk, a rice processing byproduct generated in large quantities (∼20% of the grain weight), creates a major disposal problem for the rice industry. However, rice husk contains high-value bioactive compounds that can provide potential health benefits. The objective of this study was to extract high-value phenolic compounds from rice husk using supercritical carbon dioxide (SC–CO2) technology. In this study, the effects of different extraction conditions, namely, temperature (40 and 60 °C), pressure (30 and 40 MPa), and ethanol concentration (15 and 25%, w/w) on the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (AA) were investigated. The extraction of phenolic compounds was also studied using different SC-CO2 modifiers, i.e., ethanol and ethanol-water. The highest TPC, TFC, and AA were achieved with 30 MPa, 60 °C, and 25% ethanol-water (50%, v/v) cosolvent mixture as 1.29 mg gallic acid equivalent (GAE)/g, 0.40 mg catechin equivalent (CE)/g, and 0.23 mg Trolox equivalent (TE)/g, respectively. Increasing water content up to 50% (v/v) in the cosolvent significantly improved the extraction yield. p-Coumaric, ferulic, and syringic acids were the predominant phenolic acids in the extracts obtained by cosolvent-modified SC-CO2 and methanol extractions. In addition, ethanol-water-modified SC-CO2 increased rice husk's porosity, which could be a potential pretreatment to enhance cellulose extraction. Thus, ethanol-water-modified SC-CO2 can be utilized to recover polar bioactive compounds from food processing byproducts for developing functional foods while eliminating the use of toxic organic solvents.
Keywords: Supercritical carbon dioxide, Ethanol, Water, Extraction, Rice husk, Phenolic compounds
Graphical abstract
Highlights
-
•
Cosolvent-modified supercritical carbon dioxide (SC–CO2) extractions were studied.
-
•
Phenolic compounds were extracted from rice husk using cosolvent-modified SC-CO2.
-
•
Cosolvent-modified SC-CO2 extractions were compared with methanol extractions.
-
•
The highest yield was obtained using ethanol-water (50/50, v/v) as a cosolvent.
1. Introduction
Rice (Oryza sativa), a member of the grass family, has become the world's most widely farmed and consumed crop, covering 11% of the world's cultivated land [1,2]. Recently, global rice production has reached >500 MT [2]. The rising production rates have led to the generation of an enormous amount of byproducts, primarily husk and bran, during processing. By 2030, the production of rice husk and bran is expected to exceed 200 MT, posing significant management challenges for the rice processing industry. Formerly, rice husk was either dumped into the soil or burned in an open field, leading to the release of gaseous pollutants into the environment, as well as economic and environmental issues.
Rice husk (∼20% of grain weight) has restricted applications due to undesirable properties, including high lignin content (20–25%) and high silica content [3,4]. Until now, rice husk has been used for the following major purposes: electricity [4] and fuel production [5], wastewater treatment [6], carbonization [7], animal production [8], soil fertilization [4,9] and nano silica production [10,11]. However, rice husk is rich in bioactive compounds, namely phenolic acids and flavonoids. These compounds are secondary metabolites present in the husk with multiple biological effects, including antioxidant characteristics, which could prevent lipid oxidations and play a crucial role in preventing heart diseases [12]. Specifically, rice husk contains a high amount of phenolic compounds, i.e., coumaric acid, ferulic acid, syringic acid, caffeic acid, and hydroxybenzoic acid. The antioxidative effect of rice husk was recognized as approximately two times higher than that of cranberry, four times higher than that of red grapes, and, four times higher than that of the bound fraction of whole rice [3]. The AA of free phenolics tends to be higher than the bound fraction [13]. Additionally, the free forms of phenolic acids have higher bioaccessibility [14].
Traditionally, solvent extraction has been used to extract bioactive compounds from rice husk [3,15]. For example, Vadivel and Brindha [15] used 70–75% ethanol to extract the polyphenols from rice husk. On the other hand, Gao et al. [3] extracted the free and bound phenolics from rice husk using acetone, methanol, and ethyl acetate. However, the traditional extraction methods have major drawbacks, such as the use of large amounts of toxic solvents, oxidation due to the presence of air, and the need for additional separation and purification steps [16]. Therefore, there is a need for a new extraction method to recover phenolic compounds from rice husk using only food-grade solvents.
SC-CO2 extraction is considered a safe and environmentally friendly method for extracting bioactive compounds with high selectivity and purity, and minimal degradation. CO2, an FDA-approved solvent with mild critical conditions (31.1 °C and 7.4 MPa), is non-toxic, inexpensive, abundant, and non-flammable. SC-CO2 has been mainly used to extract non-polar compounds such as triacylglycerols [17,18], phytosterols [[19], [20], [21]], and lycopene [[22], [23], [24]]. However, SC-CO2, alone being a non-polar solvent, has limited ability to extract polar compounds such as phenolic compounds. Therefore, cosolvents such as ethanol have been introduced along with SC-CO2 to modify its polarity and solvating power, providing better efficiency in extracting polar compounds. The most significant benefits of this technique are the ease with which it can separate solvents, eliminate oxidation, and prevent thermolabile bioactive compounds from degrading, therefore, maximizing the extraction yields. Previously, ethanol-modified SC-CO2 has been used in extracting phenolic compounds from various sources, including chestnut [25], Arachis Hypogea [26], and grape bagasse [27]. Further, to increase the phenolic concentration, water has been used along with ethanol to create a more polar mixture. Ethanol-water-modified SC-CO2 has increased the extraction yield of phenolic compounds from grape marc [28], purple corn cob [29], Hypericum caprifoliatum [30], blackberry bagasse [31], grape seed [32], bamboo leaves [33], sorghum bran [34] and roselle calyces [35]. However, to the best of our knowledge, there is no study on the extraction of phenolic compounds from rice husk using ethanol/water-modified SC-CO2.
Therefore, the main objective of this study was to extract phenolic compounds from rice husk using ethanol- and ethanol-water-modified SC-CO2. Specific objectives were to: (a) investigate the effects of SC-CO2 extraction conditions, namely, temperature, pressure, and cosolvent concentration on the phenolics yield, (b) determine the effect of ethanol-water ratio in the cosolvent on the phenolics yield and composition, and (c) characterize the extracts for their TPC, TFC, AA, phenolic composition, and free and bound phenolic contents. SC-CO2 extraction was compared with the traditional methanolic extraction. Lastly, the morphology of rice husk was also analyzed after the SC-CO2 extraction for future applications like nanocellulose generation.
2. Materials and methods
2.1. Materials
Rice husk was kindly provided by Riceland Foods (AR, USA). Folin-Ciocalteu phenol reagent, sodium carbonate, sodium nitrite, aluminum chloride, sodium hydroxide, glass wool, glass beads, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulphate, and hydrochloric acid were all purchased from Sigma-Aldrich (MO, USA). The organic solvents, i.e., ethanol, methanol, and hexane, were obtained from Fisher Scientific (PA, USA). Liquid CO2 (99.99% purity) and nitrogen (99.99% purity) were supplied by Airgas, Inc. (AR, USA).
2.2. SC-CO2 extraction
The SC-CO2 extractions were carried out using a lab-scale extractor (SFT-120, Supercritical Fluid Technologies Inc., DE, USA) (Fig. S1) according to the method of Ubeyitogullari and Rizvi [36]. First, the rice husk was ground and sieved through a mesh size of 1.0 mm screen. The particle size distribution of the ground rice husk was 1 mm > 60.8 ± 0.7% (w/w) > 425 μm; 425 μm > 8.2 ± 0.1% (w/w) > 250 μm; 250 μm > 20.6 ± 0.1% (w/w) > 180 μm; 180 μm > 2.7 ± 0.4% (w/w) > 150 μm; and 150 μm > 7.7 ± 0.3% (w/w). Then, 18 g of the rice husk powder, mixed with non-porous glass beads (12 g) to improve mass transfer properties, was loaded into the high-pressure vessel (100 mL with an inner diameter of 30 mm). Both ends of the vessel were sealed with glass wool to prevent blockage. Before the extraction, the system was flushed with CO2 for complete oxygen removal. The micrometering valve was heated to 80 °C to prevent freezing due to the Joule-Thomson effect. Next, the pressure and temperature were set to meet the extraction conditions along with the ethanol/water flow rate. The system was kept at these set conditions (temperature, pressure and cosolvent concentration) for 20 min static extraction time. Ethanol/water was pumped into the system using a cosolvent pump (LL-Class, Supercritical Fluid Technologies Inc., DE, USA) at predetermined flow rates to provide the required ethanol/water concentration (15 or 25%, w/w) in the vessel. When the static extraction time ends, a continuous flow of CO2 (1 L/min, measured at ambient conditions (23 °C and 0.1 MPa)) was attained by adjusting the micrometering valve. The extract was collected in a vial placed in an ice bath to prevent sample carryover and degradation. The extraction conditions were determined based on the preliminary experiments at pressures of 30–50 MPa, temperatures of 40–80 °C, and cosolvent concentrations of 10–25% (w/w). After the preliminary experiments, the ethanol-modified SC-CO2 extractions were run at different pressures (30 and 40 MPa), temperatures (40 and 60 °C), and cosolvent concentrations (15 and 25%, w/w). Different ethanol/water mixtures in various proportions (25/75, 50/50, 75/25, v/v) were investigated at the optimized extraction conditions. Finally, the extracts were flushed with nitrogen and stored at −80 °C until characterized. The collected extracts were characterized without further separation steps. The total yield was calculated by considering the amount of extract collected and the concentration of phenolic compounds in the extracts.
2.3. Conventional methanol extraction
The conventional methanolic extraction was performed according to the method of Xiong et al. [37]. This method was included to compare the different extraction methods in terms of their extraction yield and composition. In brief, 1 g of rice husk powder (mesh size 1.0 mm) was mixed with 45 mL of 80% (v/v) methanol solution in a centrifuge tube. The samples were incubated at 50 °C for 1 h with vortexing every 15 min. After 1 h incubation period, the tubes were centrifuged at 3220 rpm at 4 °C for 10 min. Next, the supernatant was collected, and the residue was again suspended in 45 mL of 80% (v/v) methanol solution to repeat the extraction for the second time. Finally, the extracts were pooled and stored at −80 °C under a blanket of nitrogen until analysis. The data was collected in triplicates and presented in the form of mean ± standard deviation.
2.4. Determination of total phenolic content (TPC)
The Folin-Ciocalteu method was used to determine the TPC using gallic acid as a standard [38]. Briefly, 100 μL of the extract was mixed with 500 μL of 0.2 N Folin-Ciocalteu's phenol reagent solution and allowed to react for 5 min at room temperature (23 °C). Further, 400 μL of 0.7 M sodium carbonate solution was added, and the mixture was incubated at room temperature (23 °C) for 2 h. The absorbance of the solution was recorded at a wavelength of 760 nm using a spectrophotometer (Milton Roy Spectronic 1201, PA, USA). The calibration curve (R2 = 0.9977) was prepared using different concentrations of gallic acid (0–200 ppm) under the same conditions. The analysis was conducted in triplicate, and the TPC was expressed as milligram gallic acid equivalent (GAE) per gram of rice husk ± standard deviation (mg GAE/g).
2.5. Determination of bound phenolics in the extracts
The extraction of bound phenolics was carried out following the method of Gao et al. [3] with slight modifications. Briefly, 1 mL of extract was allowed to digest at room temperature (23 °C) with 20 mL of 2 M NaOH and shaken for 1 h. Further, the mixture was neutralized with 4 mL of HCl, and TPC was determined, as described in Section 2.4.
2.6. Determination of total flavonoid content (TFC)
The aluminum chloride colorimetric assay was followed to determine TFC with catechin as the standard [39]. In short, 4 mL of water and 1 mL of sample were mixed properly before adding 300 μL of 5% sodium nitrite solution. After 5 min incubation, 300 μL of 10% aluminum chloride solution was added, and then, after 1 min, 2 mL of 1 M sodium hydroxide solution was included in the mixture. Next, distilled water was added to make the total volume 10 mL. Finally, the absorbance of the solution was measured at 510 nm wavelength using the same spectrophotometer described above. The calibration curve (R2 = 0.9999) was prepared using different concentrations of catechin (0–100 ppm) under similar conditions. The analysis was conducted in triplicates, and the TFC was expressed as milligram catechin equivalent (CE) per gram of rice husk ± standard deviation (mg CE/g).
2.7. Determination of antioxidant activity (AA)
The ABTS assay was used to determine the AA of the extracts, where Trolox was used as the standard (0–100 ppm) [40]. Briefly, 7 mM ABTS solution was reacted with 2.45 mM potassium persulfate solution in a 1:2 (v/v) ratio, respectively, and incubated for 8 h in the dark at room temperature (23 °C). Further, the solution was diluted with ethanol to obtain an absorbance of 0.700 ± 0.02 at 734 nm. Next, 100 μL of the extract was mixed with 2 mL of ABTS solution and incubated for 6 min. After incubation, the absorbance of the samples was recorded at 734 nm (n = 3). The calibration curve (R2 = 0.9943) was prepared using different concentrations of Trolox (10–100 ppm) under similar conditions. The data were expressed as milligram Trolox equivalent (TE) per gram of rice husk ± standard deviation (mg TE/g) using equation (1).
| (1) |
where TC (mg/mL) is the concentration of Trolox obtained using the standard curve, V is the extract volume (mL), d is the dilution factor, and m (g) is the rice husk amount used for extraction [41].
2.8. HPLC analysis of phenolic compounds
Phenolic compounds in the rice husk extracts were identified according to the method of Gao et al. [3]. The samples were analyzed using an HPLC system (SPD-20AV UV/VIS detector, SIL- 10AF autosampler, a CTO-20A column oven, Shimadzu Corp., Japan) at 280 nm. An aliquot of 10 μL was injected onto a reversed-phase C18 Symmetry column (4.6 × 250 mm, 5 μm; Waters, MA, USA). The mobile phase consisted of two solvents: solvent A (1% formic acid) and solvent B (100% acetonitrile). The mobile phase was run at a flow rate of 1.0 mL/min using the following gradient: 0–5 min 10% B, 5–20 min 25% B, 20–25 min 35% B, 25–40 min 90% B, 40–50 min 10% B, and 50–60 min 10% B. The column temperature was kept constant at 25 °C. Authentic standards of phenolic acids were used for their identification. Phenolic acids were reported as percentages of the total phenolic acids identified in the samples.
2.9. Morphology of rice husk after SC-CO2 extraction
A scanning electron microscope (FEI NovaNanolab200 Dual-Beam system) was used to determine the morphology of rice husk before and after SC-CO2 extraction. In brief, samples were coated with a gold layer using a sputter-coater (EMITECH SC7620 Sputter Coater, MA, USA). The analysis was conducted at 15 kV and 15 mA with a working distance of 5 mm under low vacuum mode.
2.10. Statistical analysis
The experiments were expressed as the mean ± standard deviation with three replicates per sample. ANOVA and Tukey's test were performed using statistical software JMP Pro 16.0 (SAS Institute, NC, USA) with a 5% significance level.
3. Results and discussion
The extraction conditions (30–40 MPa, 40–60 °C, and 15–25% (w/w) ethanol concentration) were determined according to the TPCs and TFCs of the extracts obtained in the preliminary experiments and literature [25,42,43]. The extraction time (3 h) and CO2 flow rate (1 L/min, measured at ambient conditions (23 °C and 0.1 MPa)) were adjusted based on the preliminary extraction curves (data not shown), where approximately 95% of the total phenolics and flavonoids were collected in the first 3 h of the 6 h extraction runs. In this study, ethanol-modified SC-CO2 was employed first, and the extraction conditions were optimized based on the TPC and TFC of the extracts. After optimizing the ethanol-modified SC-CO2 extractions, ethanol-water-modified SC-CO2 extraction was conducted using different ethanol-water ratios (i.e., 25/75, 50/50, 75/25, v/v) at the optimized temperature and pressure. The ethanol/water ratios were selected based on our previous study on the extraction of phenolic compounds from sorghum bran [34].
3.1. Effects of the ethanol-modified SC-CO2 extraction conditions on the TPC and TFC
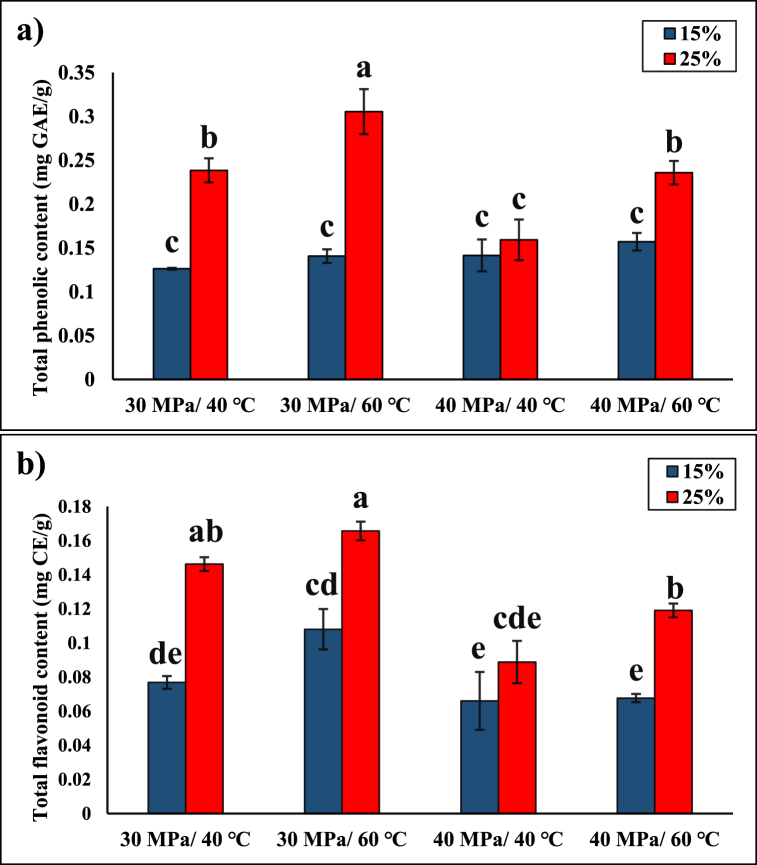
Fig. 1 shows the impacts of pressure (30 and 40 MPa), temperature (40 and 60 °C), and ethanol concentration (15 and 25%, w/w) on the TPC and TFC. Using ethanol as a cosolvent significantly improved the solvating power of SC-CO2, whereas neat SC-CO2 was not able to extract any phenolic compounds in our preliminary experiments. The highest TPC was observed at 30 MPa and 60 °C with 25% ethanol concentration as 0.36 ± 0.03 mg GAE/g, whereas the lowest TPC was obtained at 30 MPa and 40 °C with 15% ethanol concentration as 0.13 ± 0.01 mg GAE/g (Fig. 1a). The effect of cosolvent concentration on the TPC was more significant at 30 MPa compared to 40 MPa at the same conditions, where higher cosolvent concentration (25 vs. 15%) generally provided higher TPC in the extracts. A significant decrease in TPC was observed with the increase in pressure from 30 to 40 MPa (p < 0.05) when 25% cosolvent concentration was used. At the same cosolvent concentration (25%), increasing the temperature from 40 to 60 °C significantly increased the TPC of the extracts. Nevertheless, when 15% cosolvent concentration was employed, the change in pressure or temperature did not significantly influence the TPC (Fig. 1a), which could be due to the crossover pressure and the presence of cosolvent in the mixture, as described below.
Fig. 1.
Total (a) phenolic and (b) flavonoid contents of the extracts obtained via ethanol-modified SC-CO2 at different pressures, temperatures, and cosolvent ratios. Means that do not share a common letter within the same assay are significantly different (p < 0.05). GAE: Gallic Acid Equivalent, CE: Catechin Equivalent.
A similar trend was observed in the TFC yields (Fig. 1b) with different extraction conditions. The highest TFC was achieved at 30 MPa and 60 °C with 25% cosolvent concentration (0.17 ± 0.01 mg CE/g), while the lowest TFC was recorded at 40 MPa and 40 °C with 15% ethanol (0.07 ± 0.01 mg CE/g).
Pressure and temperature together dictate the solubility of solutes in SC-CO2, making it difficult to study their effects separately. In a previous SC-CO2 extraction study, the phenolic compound yield from Baccharis dracuncufolia increased with the increase in pressure and temperature [43]. However, a different trend was followed at lower pressure (10–20 MPa) and higher temperatures (40–60 °C) in ethanol-modified SC-CO2 extraction [43]. Lower pressures (10–20 MPa) resulted in higher TPC and TFC yields due to improving the penetration depth of the fluid to interact with the extractable components and lowering the fluid density [27,32,42]. Thus, the lower mass transfer (i.e., high density and viscosity along with low dispersion coefficient and penetration rate of the fluid, resulting in limited interaction with the extractable components) at the high pressure (40 MPa) may have contributed to the low phenolic yields (Fig. 1) [27]. The vapor pressure effect over the density effect using ethanol-modified SC-CO2 extraction explained the extractability of SC-CO2 in a previous study [43]. This behavior, known as the crossover isotherm, was observed around 30 MPa [43]. The temperature of the extraction plays a critical role along with the pressure in determining the extraction yields, where the effect of temperature changes depending on the crossover pressure. At constant pressure, increasing the extraction temperature reduces the solvent density but increases the vapor pressure of solute and mass transfer properties [44]. Below the crossover pressure, the solubility decreases with increasing temperature as the change in density becomes more dominant. On the other hand, above the crossover pressure, the solubility increases with increasing the temperature as the increase in the vapor pressure of the solute is predominant [36,45]. Castro-Vargas et al. [42] revealed the enhancement of solute vapor pressure at higher temperature (40 °C) and lower pressure (30 MPa) using ethanol-modified SC-CO2 extraction of phenolics from guava seeds, where they reported the crossover pressure as ∼25 MPa.
Additionally, the increase in cosolvent concentration from 15 to 25% increased the phenolic yield by improving the polarity of the solvent (Fig. 1). Ethanol, as a polar solvent with a critical point of 241 °C and 6.1 MPa [46], increases the interactions and hydrogen bonding with polar functional groups [47], resulting in higher extraction yields of polar compounds. The phenolic and flavonoid contents from rice husk were maximized at a high ethanol concentration of 25%, low pressure of 30 MPa, and high temperature of 60 °C. Similar trends were observed in the extractions of polyphenols from several materials, including chestnut bars [25], guava seed [42] and grape bagasse [27], where at higher ethanol concentrations (10%), lower pressures (10–20 MPa), and higher temperatures (40–60 °C), the extraction yields were improved. Farías-Campomanes et al. [27] also revealed the fact that high pressure (35 MPa) lowers the dispersion coefficient of SC-CO2, creating porosity in the extraction bed, which reduces the contact time between solute and solvent, lowering the mass transfer rate and the extraction yield. Similarly, Putra et al. [26] improved the phenolic and flavonoid yield at higher temperatures by improving the solvating power using ethanol-modified SC-CO2 extraction. However, Zulkafli et al. [33] revealed higher phenolic yields at 20 MPa (among 10–20 MPa) and 50 °C (among 50–95 °C) with 10% cosolvent (among 5–10%); where increasing the temperature decreased the yield because of the reduction in the density and solvating power of the fluid.
3.2. Effects of the ethanol-modified SC-CO2 extraction conditions on the AA
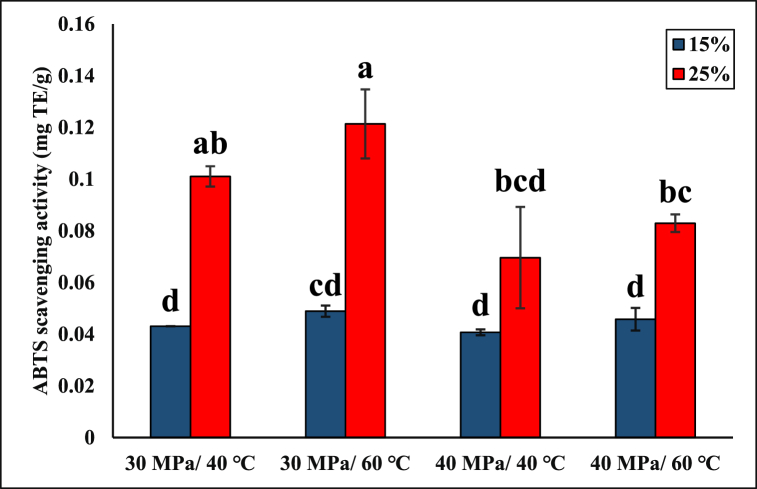
Fig. 2 depicts the antioxidant profile of extracts obtained using different pressures (30 and 40 MPa), temperatures (40 and 60 °C), and ethanol concentrations (15 and 25%). As stated before, in the preliminary experiments, pure SC-CO2 was unable to extract phenolic compounds, and consequently, these samples did not exhibit any AA. However, the addition of ethanol improved the solvating power, hence increasing the AA. The highest AA (0.12 mg TE/g) was achieved at 30 MPa, 60 °C, and 25% ethanol, while the lowest (0.04 mg TE/g) was obtained at 40 MPa, 40 °C, and 15% ethanol (p < 0.05). The change in the AA of the extracts at different pressures and temperatures was more pronounced when 25% ethanol was used instead of 15% (Fig. 2), which agreed with the TPC data (Fig. 1). As expected from the TPC data, the extracts obtained at the pressure of 30 MPa showed higher AA compared to their counterparts collected using 40 MPa. Additionally, at 40 MPa, the change in temperature from 40 to 60 °C did not significantly affect the AA (p < 0.05). Castro-Vargas et al. [42] also observed the insignificant effect of temperature (40–60 °C) at a pressure of 30 MPa in the ethanol-modified SC-CO2 extraction. Overall, the AA data followed a similar trend to the TPC results shown in Fig. 1a; thus, a higher TPC resulted in a higher AA. Likewise, Spiridon et al. [48] reported higher AA with higher TPC in the plant extract and revealed a linear relationship between the AA and TPC. A similar trend was observed in the AA performed using the DPPH assay by Butsat and Siriamornpun [49], where higher TPC provided higher AA.
Fig. 2.
The antioxidant activity of the extracts obtained using ethanol-modified SC-CO2. Means that do not share a common letter are significantly different (p < 0.05). TE: Trolox Equivalent.
3.3. Effects of the cosolvent types during SC-CO2 extraction on the TPC, TFC, and AA
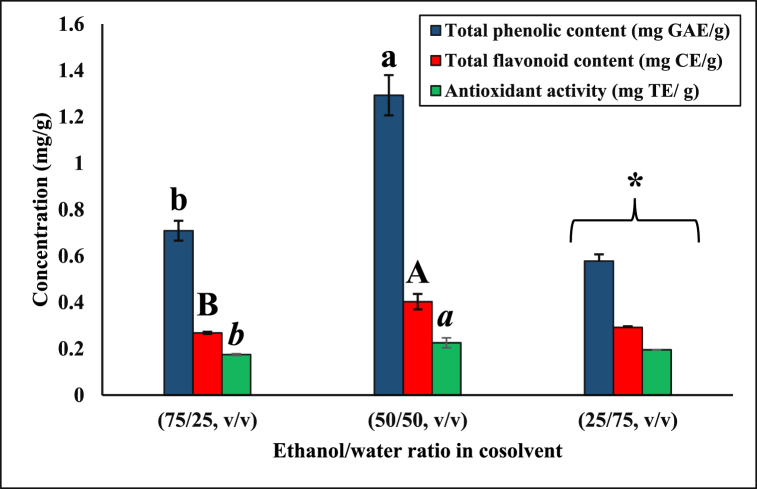
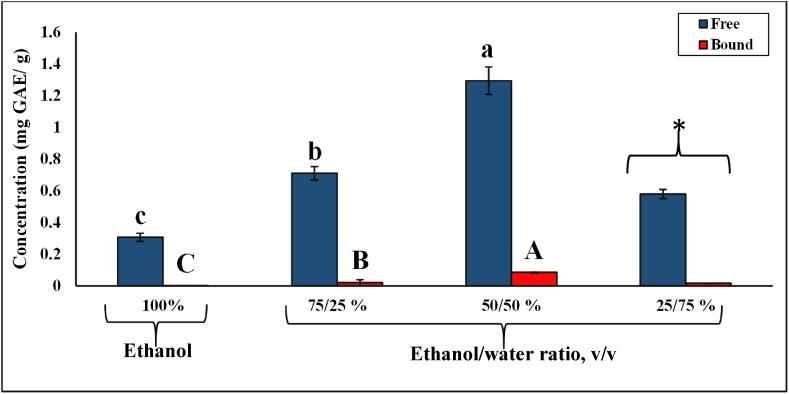
The statistically optimized conditions (30 MPa and 60 °C) with the highest TPC and TFC were selected to investigate the effect of different cosolvents on the recovery of phenolic compounds. Ethanol-water mixtures at different ratios (25/75, 50/50, 75/25, v/v) were used as cosolvents at constant pressure (30 MPa) and temperature (60 °C). The highest TPC (1.29 ± 0.09 mg GAE/g) and TFC (0.40 ± 0.03 mg CE/g) were achieved with 50/50 (v/v) ethanol-water ratio, whereas the lowest phenolic and flavonoid yields were obtained as 0.59 ± 0.03 mg GAE/g and 0.27 ± 0.01 mg CE/g with 75/25 (v/v) ethanol-water mixture, respectively (p < 0.05) (Fig. 3). The extraction conditions (30 MPa and 60 °C with 50/50 (v/v) ethanol-water cosolvent) with the highest TFC resulted in the highest AA (0.23 ± 0.02 mg TE/g) (Fig. 3).
Fig. 3.
Total phenolic, flavonoid content, and antioxidant activity using ethanol-water-modified SC-CO2 extraction at different ethanol/water ratios. *The condition was unable to operate properly at the lab-scale extractor due to clogging and blockage. Separate statistical analyses were conducted for TPC, TFC, and AA, and the means that do not share a common letter within the same characterization method (p < 0.05). GAE: Gallic Acid Equivalent, CE: Catechin Equivalent, TE: Trolox Equivalent.
When the system was run with 25/75 (v/v) ethanol-water as a cosolvent at 30 MPa and 60 °C, it was not possible to obtain a constant flow of CO2, and the flow was blocked constantly, resulting in low extraction yields. The smaller particle size within the samples improves the extraction rates by reducing the diffusion path of solute and increasing specific interfacial area; however, the smaller particle size tends to form clumps, reducing the fluidized bed viscosity and extraction efficiency by clogging the filters [50,51]. Another reason for this clogging problem could be due to the increased solubility of other macromolecules in rice husk with high water content [52]. Due to these issues during the extraction, 100% water was not investigated as a cosolvent.
Ethanol-water as a cosolvent helps release and solubilize the polar compounds via breaking the chemical bonds by increasing the acidification and reducing the solvent's selectivity [53]. Paes et al. [53] reported the formation of two phases when a 50% ethanol-water cosolvent mixture was used; however, 10% cosolvent was reported to improve solubility and, in turn, the extract yield. Water as a cosolvent enhances hydrogen bond's breakdown to solubilize phenols and improves mass transport via molecular diffusion [52]. Although using water in the cosolvent may form two phases, i.e., supercritical and liquid, the resulting solvent mixture can help solubilize more polar compounds [53]. Water solubility improves with the addition of ethanol into SC-CO2 due to the strong hydrogen bonding between ethanol and water [54]. Ravetti Duran et al. [55] observed even a small fraction of water present in the ternary mixture (i.e., CO2, ethanol, and water) promotes the formation of a two-phase system. Therefore, in this study, the presence of SC-CO2+ethanol + water mixture phase and ethanol + water liquid phase was expected [55]. Overall, the phenolic yield achieved via ethanol-water (50%, v/v) mixture (1.29 ± 0.09 mg GAE/g) was significantly higher than that obtained using pure ethanol (0.36 ± 0.03 mg GAE/g) as a cosolvent at the same extraction conditions (30 MPa, 60 °C, and 25% cosolvent). Similarly, in a previous study, water as a cosolvent further improved the solubility power of SC-CO2 by interacting with polar compounds in purple corn cob and provided a higher yield of phenolics as the water ratio increased in the ethanol-water mixture [29]. In addition, Zulkafli et al. [33] revealed that phenolic extraction yield and the AA of the extracts from bamboo leaves were improved when 25/75 (v/v) ethanol-water-modified SC-CO2 extraction was used. Paes et al. [53] also improved the TPC and AA of blueberry extracts by using an ethanol-water mixture in SC-CO2. Additionally, they reported that higher cosolvent concentrations (e.g., >50%) resulted in the two-phase formation, lowering the extraction yield. On the other hand, Monroy et al. [29] obtained the maximum polyphenol yield using an ethanol/water mixture as a cosolvent in ethanol-water-modified SC-CO2 extraction (400 bar, 50 °C, 32–35% cosolvent mixture).
3.4. Comparison of methanol and ethanol-water-modified SC-CO2 extractions
The TPC, TFC, and AA of the extracts obtained at the optimized ethanol-water-modified SC-CO2 extraction (30 MPa, 60 °C, and 25% ethanol-water (50%, v/v)) were compared with the conventional methanolic extraction. The TPC, TFC, and AA obtained using ethanol-water-modified SC-CO2 extraction at the optimized conditions were significantly lower than those obtained via methanol extraction (p < 0.05). The methanolic extraction produced extracts with free phenolics of 1.92 ± 0.07 mg GAE/g, free flavonoids of 1.12 ± 0.14 mg CE/g, and AA of 2.58 ± 0.06 mg TE/g. In a previous study, the free phenolics and flavonoids in the extracts obtained using methanolic extraction from rice husk were reported as 1.20 ± 0.06 mg GAE/g and 0.73 ± 0.07 mg CE/g, respectively [3]. In addition, the TPC of the rice husk methanolic extracts changed from 1.2 to 2.2 mg GAE/g depending on the rice growth site [49]. The variation in the TPC and TFC in the extracts obtained using methanol extraction could be due to the differences in the rice husk source and extraction steps followed [3]. Butsat et al. [56] reported a TPC of 1.3 mg GAE/g in rice husk at the fully ripe grain stage and observed the highest phenolic content of 2.1 mg GAE/g during the flowering stage of the grain development. Overall, compared to methanol extraction, ethanol-water-modified SC-CO2 was effective in extracting free phenolic and flavonoids when the solvent-to-sample (w:w) ratio (71:1 in methanol extraction vs. ∼20:1 in cosolvent-modified SC-CO2 extraction) was considered. In terms of industrial applications, the proposed SC-CO2 approach can reduce the use of toxic organic solvents, prevent oxidation during extraction, and protect thermolabile compounds. These advantages can help offset the need for the capital cost. Additionally, these pressure and temperature conditions reported here are relatively easier to achieve and operate at large scale [27,57,58].
Furthermore, the bound phenolics in the extracts obtained via ethanol-and ethanol-water-modified SC-CO2 were determined (Fig. 4). The concentration of bound phenolics obtained using ethanol-modified SC-CO2 extraction (∼0.001 mg GAE/g) was significantly lower than that extracted via ethanol-water-modified SC-CO2 (0.08 mg GAE/g). As the water concentration increased in the cosolvent mixture, the bound phenolic yield also improved; nevertheless, as discussed before, at 25/75 (v/v) ethanol/water ratio, it was not possible to operate the extractor effectively (Fig. 5). Therefore, water was able to improve the solubilization of bound phenolics by increasing the contact surface area between solvent and solute [59,60]. In the literature, the bound phenolics and flavonoids were reported as 13.70 ± 0.67 mg GAE/g and 2.35 ± 0.12 mg CE/g, respectively, when methanolic extraction was employed [3]. Butsat et al. [56] reported the presence of bound phenolics ranging from 6.6 to 8.0 mg GAE/g during five stages of Thai rice development.
Fig. 4.
Free and bound phenolics in the extracts obtained using SC-CO2 with different cosolvent ratios. * The condition was unable to operate properly at the lab-scale extractor due to clogging and blockage. The free and bound fractions were statistically compared separately, and the means that do not share a common letter within the same characterization method are significantly different (p < 0.05).
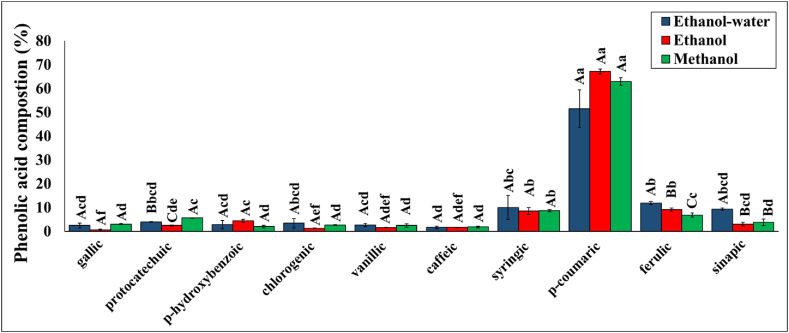
Fig. 5.
Phenolic acid composition of the extracts obtained using different extraction methods.
Means with different capital letters within the same phenolic acid group and means with different lowercase letters within the same extraction method are significantly different (p < 0.05).
Phenolic acids have been widely used to prevent carcinogenesis and mutagenesis and help reduce the incidence of several chronic diseases [[61], [62], [63]]. The phenolic groups present in the rice husk exist in both free and bound forms. In this study, even though the ethanol-water-modified SC-CO2 extraction extracted more bound phenolics compared to ethanol-modified SC-CO2, it still primarily isolated free forms of phenolic acids (1.29 mg GAE/g free phenolics vs. 0.08 mg GAE/g bound phenolics). On the other hand, methanolic extraction extracted both free and bound phenolics. According to the bioaccessibility and bioavailability studies of phenolic acids, the bound phenolics were unable to be hydrolyzed by the human digestive system, and they were released in the colon tract by the action of bacterial enzymes [64]. Hole et al. [14] reported low bioavailability of bound phenolics along with their poor biological activity; however, their bioaccessibility and bioavailability improved by increasing the concentration of free phenolics in the cereal-based products. Therefore, the recovery of free phenolic acids via ethanol-water-modified SC-CO2 may provide advantages in developing highly bioavailable formulations for enhancing human health.
3.5. Phenolic acid composition
Figs. S2 and S3 depict representative HPLC chromatographs of phenolic acids extracted from rice husk and some standards. Phenolic acid compositions of the extracts obtained via ethanol-modified SC-CO2, ethanol-water-modified SC-CO2 extraction, and methanol extraction (control) were also determined (Fig. 5). The identified phenolic acids were gallic acid, protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, and sinapic acid, which agrees with the phenolic acid composition in rice husk reported by Gao et al. [3]. The p-coumaric acid had the highest concentration among all identified phenolic acids in all the samples. Ferulic and sinapic acid ratios in the extracts obtained via ethanol-water-modified SC-CO2 were significantly higher than those obtained with methanolic extraction (p < 0.05). However, protocatechuic acid was present in higher percentages in the extract obtained with methanolic extraction compared to the ones collected with cosolvent-modified SC-CO2 extractions. Other than these phenolic acid ratios, all other phenolic acid percentages were similar in all extraction methods (p > 0.05). In addition to the calorimetric methods and phenolic acids’ identification, the identification of individual flavonoids using liquid chromatography can be helpful for the specific applications of these extracts. Such analysis can provide more information about the specific flavonoids contributing to the TFC measured via the aluminum chloride colorimetric method.
3.6. Effect of the SC-CO2 extraction on the morphology of rice husk
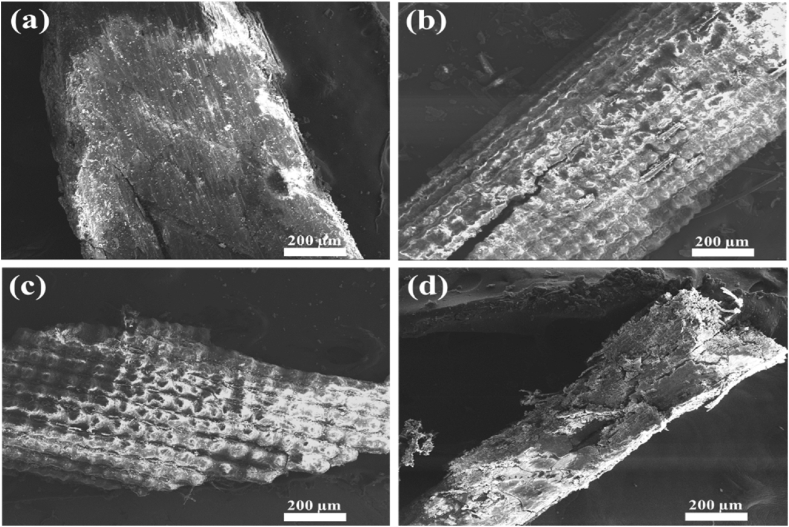
Fig. 6 shows the SEM images of untreated rice husk and the ones treated with different extraction conditions. The untreated rice husk had a flat surface with minimum porosity (Fig. 6a), where the surface was covered with waxes and silica. Park et al. [65] also revealed a similar surface structure of rice husk. On the other hand, when rice husk was treated with pure SC-CO2 (Fig. 6b) or ethanol-modified SC-CO2 (Fig. 6c), there were significant changes on the surface of the husk. The increased irregular surface with some porosity could be due to the extraction of waxes from the surface Otto et al. [66]. When ethanol-water-modified SC-CO2 was used, the structure was changed entirely (Fig. 6d). As discussed above, this extraction method provided the highest yield of TPC and TFC. In addition, water may have also dissolved other macromolecules from rice husk [52], resulting in a more open porous structure. This improved porosity could enhance the extraction of cellulose, and make the generation of nanocellulose easier. Various studies utilized rice husk to extract cellulose and form nanocellulose, expanding the application of this byproduct in the food and pharmaceutical industries [3,[67], [68], [69]].
Fig. 6.
SEM images of (a) untreated rice husk and rice husks treated with (b) pure SC-CO2, (c) ethanol-modified SC-CO2, and (d) ethanol-water-modified SC-CO2.
4. Conclusions
In this study, phenolic compounds were extracted from rice husk using a green and sustainable approach based on SC-CO2 extraction. Extraction conditions were investigated and optimized for the highest total phenolic and flavonoid yields. Compared to ethanol-modified SC-CO2, ethanol-water-modified SC-CO2 resulted in higher TPC and TFC. The optimized extraction conditions were 30 MPa and 60 °C with 25% ethanol-water (50%, v/v) as a cosolvent, resulting in 1.29 mg GAE/g of TPC, 0.40 mg CE/g of TFC, and 0.23 mg TE/g of AA. Increasing the water content to 50% (v/v) in the cosolvent significantly improved the total phenolic and flavonoid yields. Even though methanolic extraction resulted in higher TPC (1.92 mg GAE/g), TFC (1.12 mg CE/g), and AA (2.58 mg TE/g), it used a higher solvent-to-solid ratio along with toxic solvents limiting its food applications. Most of the phenolics extracted via SC-CO2 were in their free form, potentially providing higher bioavailability. The major phenolic acids in the extracts were p-coumaric, ferulic, and syringic. Ethanol-water-modified SC-CO2 extraction increased the porosity of the husk; therefore, this extraction method could be used as a pretreatment to increase the efficiency of cellulose extraction. Overall, this green process uses only food-grade materials to recover antioxidants from rice husk that can be utilized in developing functional foods as well as new products in the pharmaceutical and cosmetic industries. This process has the potential to be scaled up for industrial-scale applications.
Author contribution statement
Sumanjot Kaur: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Ali Ubeyitogullari: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Ali Ubeyitogullari was supported by the National Institute of Food and Agriculture [Multistate Project NC1023, Accession number 1025907]. Sumanjot Kaur was supported by University of Arkansas, Graduate Student Professional Congress (GPSC) research grant. The APC was funded by the Open Access Publishing Fund administered through the University of Arkansas Libraries.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Acknowledgements
We greatly appreciate the use of the Arkansas Materials Characterization Facility for the SEM analysis. We also thank Dr. Jin-Woo Kim and Dr. Ya-Jane Wang for valuable discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14196.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Khush G.S. What it will take to Feed 5.0 Billion Rice consumers in 2030. Plant Mol. Biol. 2005;59:1–6. doi: 10.1007/s11103-005-2159-5. [DOI] [PubMed] [Google Scholar]
- 2.FAO. FAO . 2022. Cereal Supply and Demand Brief.https://www.fao.org/worldfoodsituation/csdb/en/ [cited 2022 April]; Available from: [Google Scholar]
- 3.Gao Y., Guo X., Liu Y., Fang Z., Zhang M., Zhang R., You L., Li T., Liu R.H. A full utilization of rice husk to evaluate phytochemical bioactivities and prepare cellulose nanocrystals. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-27635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S., Sangwan P., Dhankhar R.M.V., Bidra S. Utilization of rice husk and their ash: a review. Res. J. Chem. Env. Sci. 2013;1:126–129. [Google Scholar]
- 5.Pradhan A., Ali S., Dash R. Biomass gasification by the use of rice husk gasifier. Special Issue of International Journal on Advanced Computer Theory and Engineering (IJACTE) 2013;2:14–17. [Google Scholar]
- 6.Hossain N., Nizamuddin S., Griffin G., Selvakannan P., Mubarak N.M., Mahlia T.M.I. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltani N., Bahrami A., Pech-Canul M.I., González L.A. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015;264:899–935. doi: 10.1016/j.cej.2014.11.056. [DOI] [Google Scholar]
- 8.Sjofjan O., Adli D.N., Djunaidi I., Kuncoro K. Utilization of biogas liquid waste for starter in the fermentation of rice husk as a potential feed for poultry. Anim. Prod. 2020;22:24–30. doi: 10.20884/1.jap.2020.22.1.38. [DOI] [Google Scholar]
- 9.Badar R., Qureshi S.A. Composted rice husk improves the growth and biochemical parameters of sunflower plants. Journal of Botany. 2014;2014 doi: 10.1155/2014/427648. [DOI] [Google Scholar]
- 10.Liou T.-H., Yang C.-C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater. Sci. Eng., B. 2011;176:521–529. doi: 10.1016/j.mseb.2011.01.007. [DOI] [Google Scholar]
- 11.Gu S., Zhou J., Yu C., Luo Z., Wang Q., Shi Z. A novel two-staged thermal synthesis method of generating nanosilica from rice husk via pre-pyrolysis combined with calcination. Ind. Crop. Prod. 2015;65:1–6. doi: 10.1016/j.indcrop.2014.11.045. [DOI] [Google Scholar]
- 12.Karimi E., Mehrabanjoubani P., Keshavarzian M., Oskoueian E., Jaafar H.Z.E., Abdolzadeh A. Identification and quantification of phenolic and flavonoid components in straw and seed husk of some rice varieties (Oryza sativa L.) and their antioxidant properties. J. Sci. Food Agric. 2014;94:2324–2330. doi: 10.1002/jsfa.6567. [DOI] [PubMed] [Google Scholar]
- 13.Sumczynski D., Kotásková E., Družbíková H., Mlček J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016;211:339–346. doi: 10.1016/j.foodchem.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 14.Hole A.S., Rud I., Grimmer S., Sigl S., Narvhus J., Sahlstrøm S. Improved bioavailability of dietary phenolic acids in whole grain barley and oat groat following fermentation with Probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012;60:6369–6375. doi: 10.1021/jf300410h. [DOI] [PubMed] [Google Scholar]
- 15.Vadivel V., Brindha P. Antioxidant property of solvent extract and acid/alkali hydrolysates from rice hulls. Food Biosci. 2015;11:85–91. doi: 10.1016/j.fbio.2015.06.002. [DOI] [Google Scholar]
- 16.Peanparkdee M., Iwamoto S. Bioactive compounds from by-products of rice cultivation and rice processing: extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019;86:109–117. doi: 10.1016/j.tifs.2019.02.041. [DOI] [Google Scholar]
- 17.Cornelio-Santiago H.P., Gonçalves C.B., de Oliveira N.A., de Oliveira A.L. Supercritical CO2 extraction of oil from green coffee beans: solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling. J. Supercrit. Fluids. 2017;128:386–394. doi: 10.1016/j.supflu.2017.05.030. [DOI] [Google Scholar]
- 18.Jokic S., Rezica S., Svilović S., Vidovic S., Bilic M., Velić D., Jurković V. Fatty acid composition of the oil obtained from soybeans by extraction with supercritical carbon dioxide. Czech J. Food Sci. 2013;31:116–125. doi: 10.17221/8/2012-CJFS. [DOI] [Google Scholar]
- 19.Hrabovski N., Sinadinović-Fišer S., Nikolovski B., Sovilj M., Borota O. Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur. J. Lipid Sci. Technol. 2012;114:1204–1211. doi: 10.1002/ejlt.201200009. [DOI] [Google Scholar]
- 20.Eller F.J., Moser J.K., Kenar J.A., Taylor S.L. Extraction and analysis of tomato seed oil. J. Am. Oil Chem. Soc. 2010;87:755–762. doi: 10.1007/s11746-010-1563-4. [DOI] [Google Scholar]
- 21.Nyam K.L., Tan C.P., Lai O.M., Long K., Che Man Y.B. Optimization of supercritical CO2 extraction of phytosterol-enriched oil from Kalahari melon seeds. Food Bioprocess Technol. 2011;4:1432–1441. doi: 10.1007/s11947-009-0253-4. [DOI] [Google Scholar]
- 22.Ubeyitogullari A., Ciftci O.N. Enhancing the bioaccessibility of lycopene from tomato processing byproducts via supercritical carbon dioxide extraction. Curr. Res. Food Sci. 2022;5:553–563. doi: 10.1016/j.crfs.2022.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobre B.P., Palavra A.F., Pessoa F.L.P., Mendes R.L. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 2009;116:680–685. doi: 10.1016/j.foodchem.2009.03.011. [DOI] [Google Scholar]
- 24.Yi C., Shi J., Xue S.J., Jiang Y., Li D. Effects of supercritical fluid extraction parameters on lycopene yield and antioxidant activity. Food Chem. 2009;113:1088–1094. doi: 10.1016/j.foodchem.2008.08.083. [DOI] [Google Scholar]
- 25.Díaz-Reinoso B., Moure A., Domínguez H. Ethanol-modified supercritical co2 extraction of chestnut burs antioxidants. Chem Eng. Process. - Process Intensification. 2020;156 doi: 10.1016/j.cep.2020.108092. [DOI] [Google Scholar]
- 26.Putra N.R., Rizkiyah D.N., Machmudah S., Shalleh L.M., Che Yunus M.A. Recovery and solubility of flavonoid and phenolic contents from Arachis Hypogea in supercritical carbon dioxide assisted by ethanol as cosolvent. J. Food Process. Preserv. 2020;44 doi: 10.1111/jfpp.14768. [DOI] [Google Scholar]
- 27.Farías-Campomanes A.M., Rostagno M.A., Meireles M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: yield, extract composition and economic evaluation. J. Supercrit. Fluids. 2013;77:70–78. doi: 10.1016/j.supflu.2013.02.006. [DOI] [Google Scholar]
- 28.Da Porto C., Decorti D., Natolino A. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: a comparison and a proposal. J. Supercrit. Fluids. 2014;87:1–8. doi: 10.1016/j.supflu.2013.12.019. [DOI] [Google Scholar]
- 29.Monroy Y.M., Rodrigues R.A.F., Sartoratto A., Cabral F.A. Influence of ethanol, water, and their mixtures as co-solvents of the supercritical carbon dioxide in the extraction of phenolics from purple corn cob (Zea mays L.) J. Supercrit. Fluids. 2016;118:11–18. doi: 10.1016/j.supflu.2016.07.019. [DOI] [Google Scholar]
- 30.Almeida R.N., Neto R.G., Barros F.M.C., Cassel E., von Poser G.L., Vargas R.M.F. Supercritical extraction of Hypericum caprifoliatum using carbon dioxide and ethanol+water as co-solvent. Chem. Eng. Process: Process Intensif. 2013;70:95–102. doi: 10.1016/j.cep.2013.05.002. [DOI] [Google Scholar]
- 31.Pasquel Reátegui J.L., Machado A.P.d.F., Barbero G.F., Rezende C.A., Martínez J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids. 2014;94:223–233. doi: 10.1016/j.supflu.2014.07.019. [DOI] [Google Scholar]
- 32.Da Porto C., Natolino A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): study on process variables and kinetics. J. Supercrit. Fluids. 2017;130:239–245. doi: 10.1016/j.supflu.2017.02.013. [DOI] [Google Scholar]
- 33.Zulkafli Z.D., Wang H., Miyashita F., Utsumi N., Tamura K. Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasa palmata) J. Supercrit. Fluids. 2014;94:123–129. doi: 10.1016/j.supflu.2014.07.008. [DOI] [Google Scholar]
- 34.Tuhanioglu A., Ubeyitogullari A. Extraction of high-value lipids and phenolic compounds from sorghum bran via a sequential supercritical carbon dioxide approach. ACS Food Sci. Technol. 2022;2:1879–1887. doi: 10.1021/acsfoodscitech.2c00266. [DOI] [Google Scholar]
- 35.Idham Z., Putra N.R., Aziz A.H.A., Zaini A.S., Rasidek N.A.M., Mili N., Yunus M.A.C. Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J. CO2 Util. 2022;56 doi: 10.1016/j.jcou.2021.101839. [DOI] [Google Scholar]
- 36.Ubeyitogullari A., Rizvi S.S.H. Production of high-purity phospholipid concentrate from buttermilk powder using ethanol-modified supercritical carbon dioxide. J. Dairy Sci. 2020;103:8796–8807. doi: 10.3168/jds.2020-18697. [DOI] [PubMed] [Google Scholar]
- 37.Xiong Y., Zhang P., Luo J., Johnson S., Fang Z. Effect of processing on the phenolic contents, antioxidant activity and volatile compounds of sorghum grain tea. J. Cereal. Sci. 2019;85:6–14. doi: 10.1016/j.jcs.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Wang H., Guo X., Hu X., Li T., Fu X., Liu R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.) Food Chem. 2017;217:773–781. doi: 10.1016/j.foodchem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 40.Mushtaq M., Sultana B., Anwar F., Adnan A., Rizvi S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids. 2015;104:122–131. doi: 10.1016/j.supflu.2015.05.020. [DOI] [Google Scholar]
- 41.Xiao F., Xu T., Lu B., Liu R. Guidelines for antioxidant assays for food components. Food Front. 2020;1:60–69. doi: 10.1002/fft2.10. [DOI] [Google Scholar]
- 42.Castro-Vargas H.I., Rodríguez-Varela L.I., Ferreira S.R.S., Parada-Alfonso F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and co-solvents. J. Supercrit. Fluids. 2010;51:319–324. doi: 10.1016/j.supflu.2009.10.012. [DOI] [Google Scholar]
- 43.Piantino C.R., Aquino F.W.B., Follegatti-Romero L.A., Cabral F.A. Supercritical CO2 extraction of phenolic compounds from Baccharis dracunculifolia. J. Supercrit. Fluids. 2008;47:209–214. doi: 10.1016/j.supflu.2008.07.012. [DOI] [Google Scholar]
- 44.Vasapollo G., Longo L., Rescio L., Ciurlia L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids. 2004;29:87–96. doi: 10.1016/S0896-8446(03)00039-1. [DOI] [Google Scholar]
- 45.Güçlü Ü., Temelli F. Correlating the solubility behavior of fatty acids, mono-, di-, and triglycerides, and fatty acid esters in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2000;39:4756–4766. doi: 10.1021/ie0001523. [DOI] [Google Scholar]
- 46.Zeng D., Li R., Jin T., Fang T. Calculating the thermodynamic characteristics and chemical equilibrium of the stepwise transesterification of triolein using supercritical lower alcohols. Ind. Eng. Chem. Res. 2014;53:7209–7216. doi: 10.1021/ie402811n. [DOI] [Google Scholar]
- 47.Díaz-Reinoso B., Moure A., Domínguez H., Parajó J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006;54:2441–2469. doi: 10.1021/jf052858j. [DOI] [PubMed] [Google Scholar]
- 48.Spiridon I., Bodirlau R., Teaca C.-A. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent. Eur. J. Biol. 2011;6:388–396. doi: 10.2478/s11535-011-0028-6. [DOI] [Google Scholar]
- 49.Butsat S., Siriamornpun S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010;119:606–613. doi: 10.1016/j.foodchem.2009.07.001. [DOI] [Google Scholar]
- 50.Putra N.R., Rizkiyah D.N., Zaini A.S., Yunus M.A.C., Machmudah S., Idham Z.b., Hazwan Ruslan M.S. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018;42 doi: 10.1111/jfpp.13689. [DOI] [Google Scholar]
- 51.Essien S.O., Young B., Baroutian S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020;97:156–169. doi: 10.1016/j.tifs.2020.01.014. [DOI] [Google Scholar]
- 52.Radzali S.A., Markom M., Saleh N.M. Co-solvent selection for supercritical fluid extraction (SFE) of phenolic compounds from labisia pumila. Molecules. 2020;25 doi: 10.3390/molecules25245859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paes J., Dotta R., Barbero G.F., Martínez J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids. 2014;95:8–16. doi: 10.1016/j.supflu.2014.07.025. [DOI] [Google Scholar]
- 54.De Marco I., Reverchon E. Supercritical carbon dioxide+ethanol mixtures for the antisolvent micronization of hydrosoluble materials. Chem. Eng. J. 2012;187:401–409. doi: 10.1016/j.cej.2012.01.135. [DOI] [Google Scholar]
- 55.Ravetti Duran R., Escudero Falsetti P., Muhr L., Privat R., Barth D. Phase equilibrium study of the ternary system CO2 + H2O + ethanol at elevated pressure: thermodynamic model selection. Application to supercritical extraction of polar compounds. J. Supercrit. Fluids. 2018;138:17–28. doi: 10.1016/j.supflu.2018.03.016. [DOI] [Google Scholar]
- 56.Butsat S., Weerapreeyakul N., Siriamornpun S. Changes in phenolic acids and antioxidant activity in Thai rice husk at five growth stages during grain development. J. Agric. Food Chem. 2009;57:4566–4571. doi: 10.1021/jf9000549. [DOI] [PubMed] [Google Scholar]
- 57.Vardanega R., Carvalho P.I.N., Albarelli J.Q., Santos D.T., Meireles M.A.A. Techno-economic evaluation of obtaining Brazilian ginseng extracts in potential production scenarios. Food Bioprod. Process. 2017;101:45–55. doi: 10.1016/j.fbp.2016.10.010. [DOI] [Google Scholar]
- 58.Carvalho P.I.N., Osorio-Tobón J.F., Rostagno M.A., Petenate A.J., Meireles M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids. 2015;105:44–54. doi: 10.1016/j.supflu.2015.03.020. [DOI] [Google Scholar]
- 59.Lee L.-S., Lee N., Kim Y.H., Lee C.-H., Hong S.P., Jeon Y.-W., Kim Y.-E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules. 2013;18:13530–13545. doi: 10.3390/molecules181113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L., Lin X., Zhang J., Zhang W., Hu X., Li W., Li C., Liu S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crop. Prod. 2019;135:1–9. doi: 10.1016/j.indcrop.2019.04.003. [DOI] [Google Scholar]
- 61.Kawabata K., Yamamoto T., Hara A., Shimizu M., Yamada Y., Matsunaga K., Tanaka T., Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/S0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 62.Chang C.J., Chiu J.H., Tseng L.M., Chang C.H., Chien T.M., Wu C.W., Lui W.Y. Modulation of HER2 expression by ferulic acid on human breast cancer MCF7 cells. Eur. J. Clin. Invest. 2006;36:588–596. doi: 10.1111/j.1365-2362.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan M., Sudheer A.R., Menon V.P. Ferulic acid: therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saura-Calixto F. Dietary fiber as a carrier of dietary antioxidants: an essential physiological function. J. Agric. Food Chem. 2011;59:43–49. doi: 10.1021/jf1036596. [DOI] [PubMed] [Google Scholar]
- 65.Park B.-D., Wi S.G., Lee K.H., Singh A.P., Yoon T.-H., Kim Y.S. Characterization of anatomical features and silica distribution in rice husk using microscopic and micro-analytical techniques. Biomass Bioenergy. 2003;25:319–327. doi: 10.1016/S0961-9534(03)00014-X. [DOI] [Google Scholar]
- 66.Otto G.P., Moisés M.P., Carvalho G., Rinaldi A.W., Garcia J.C., Radovanovic E., Fávaro S.L. Mechanical properties of a polyurethane hybrid composite with natural lignocellulosic fibers. Compos. B Eng. 2017;110:459–465. doi: 10.1016/j.compositesb.2016.11.035. [DOI] [Google Scholar]
- 67.Chen Y., Zhang L., Yang Y., Pang B., Xu W., Duan G., Jiang S., Zhang K. Recent progress on nanocellulose aerogels: preparation, modification, composite fabrication, applications. Adv. Mater. 2021;33 doi: 10.1002/adma.202005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nascimento P., Marim R., Carvalho G., Mali S. Nanocellulose produced from rice hulls and its effect on the properties of biodegradable starch films. Mater. Res. 2016;19 doi: 10.1590/1980-5373-MR-2015-0423. [DOI] [Google Scholar]
- 69.Rashid S., Dutta H. Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind. Crop. Prod. 2020;154 doi: 10.1016/j.indcrop.2020.112627. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.