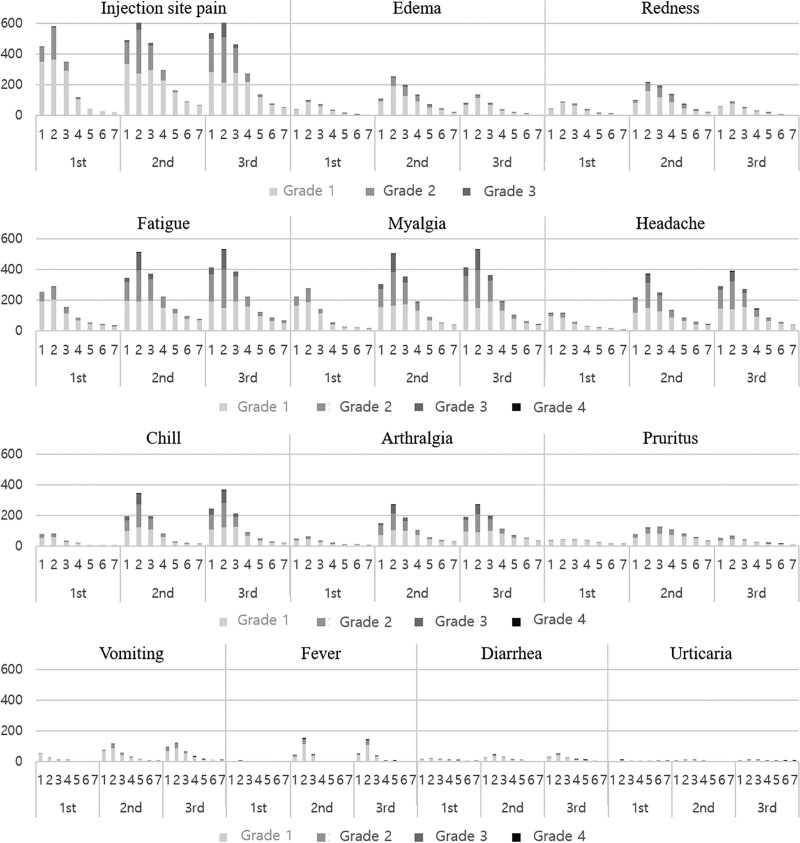
Figure 2.
Severity of adverse events for 7 days after 1st, 2nd, and 3rd dose of BNT162b2 mRNA COVID-19 vaccine. Figure 2. shows the change by date of each adverse event in 697 respondents. With most adverse events, the 2nd day was when the most adverse events were reported. The reports of adverse events decreased thereafter. COVID-19 = coronavirus disease 2019, mRNA = messenger ribonucleic acid.