Abstract
Rising adverse impact of climate change caused by anthropogenic activities is calling for advanced methods to reduce carbon dioxide emissions. Here, we review adsorption technologies for carbon dioxide capture with focus on materials, techniques, and processes, additive manufacturing, direct air capture, machine learning, life cycle assessment, commercialization and scale-up.
Keywords: Carbon dioxide capture, Climate change mitigation, Biogas upgrading, Sustainability, Adsorption technology
Introduction
Recently, the world faces with a dual global challenge including global warming derived from greenhouse gases emission as a consequence of industrialization and fossil fuel consumption, and also escalating level of energy consumption as a result of the exponential population growth (TakhtRavanchi et al. 2011; Christopher 2011). Accordingly, lately, significant efforts have been made for carbon capture and storage from the stationary sources as well as developing renewable sources of energy, such as biogas to reduce the dependency on the fossil fuels (Yu et al. 2017; Wang et al. 2018; Scarlat et al. 2018).
To this end, several different technologies have been evaluated including absorption with aqueous amine solutions, adsorption using solid sorbents, cryogenic and membranes. However, adsorption technology regarding the efficiency, eco-friendly impacts, simplicity and productivity is among the most favorable ones, while still there is a long way to pass for fully developed in the industrial scale (Xie and Suh 2013; TakhtRavanchi and Sahebdelfar 2014, 2021). Accordingly in this work, the last topics related to the carbon capture for post-combustion and biogas upgrading processes have been highlighted.
It is worth noting, carbon dioxide capture from flue gas and biogas upgrading possess an identical process with some differences in carbon dioxide percentage in the gas mixture and the operating conditions whereas the biogas as a clean source of energy, newly, received a notable interest (Baena-Moreno et al. 2019; Osman et al. 2020; Farghali et al. 2022). On the grounds, in this review paper, the most recent advances on the adsorption technology for such purposes have been demonstrated in a holistic and comprehensive way to the scientific community.
Global warming and climate change
The United Nations defines the climate change as the long-term shifts in earth’s temperature and weather pattern, caused by natural or human-made phenomena (United Nations 2022). The United Nations reported that since 1800s, the human activities have had a major role in climate change in a way that the earth is 1.1 °C warmer than 1800 (United Nations 2022). According to data published by National Aeronautics and Space Administration (NASA), the global surface temperature of earth has increased drastically in the last two decades (NASAClimate 2022a). Figure 1 represents the Global Land–Ocean Temperature Index during the last two centuries. As can be found, a significant increase in earth temperature has begun since 1980 (NASAClimate 2022a).
Fig. 1.
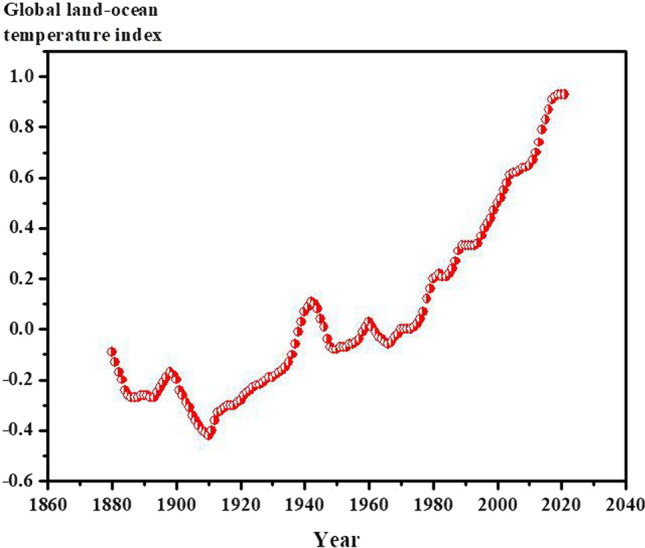
The global land–ocean temperature index for evaluating the average enhancement of annual global temperature of earth from 1880. Data provided by NASA (NASAClimate 2022a)
Hazardous human activities for meeting the worldwide energy demand are the consequence of global population growth, development of industries and lifestyle transformation. Obviously, the increasing rate of energy demand has eventuated to more consumption of different energy resources, especially fossil fuels (Shirzad et al. 2019b). As indicated in Fig. 2, the global energy consumption has rapidly increased during the last decades, which majorly attributed to the oil, gas, and coal consumption (OurWorldinData 2022). Fossil-fueled energy units exhibit tons of greenhouse gas emissions to the atmosphere (Ahmed et al. 2021).
Fig. 2.
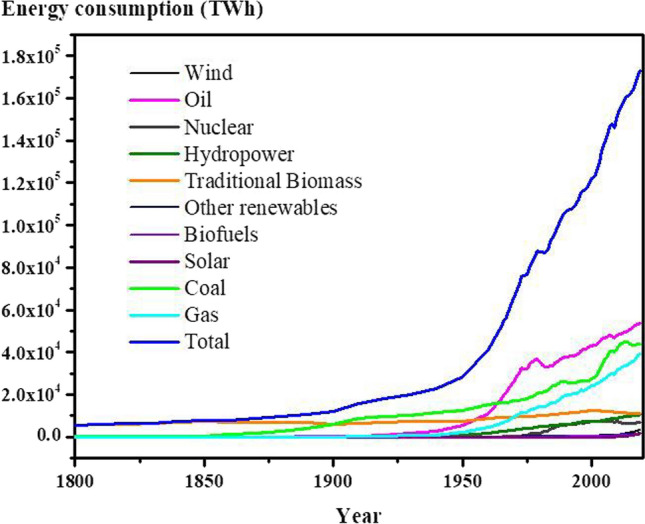
Trends of global energy consumption of oil, gas, coal, solar, biofuels, biomass, hydropower, nuclear, wind and other renewable sources during the last two century (OurWorldinData 2022)
Accordingly, carbon dioxide, methane, nitrous oxides, chlorofluorocarbons, hydrochlorofluorocarbons, and hydrofluorocarbons are considered as the major greenhouse gases, which directly attribute to the global warming (IEA 2022). It is worth noting that global energy demand decreased about 4% in 2020 due to COVID-19 pandemic compared to 2019; but at the end of 2021, the global energy demand increased 0.5% compared to the before the pandemic level (IEA 2022).
Based on the Annual Greenhouse Gas Index (AGGI) combined warming effect of all greenhouse gases compared to the condition in 1990, after the industrial revolution, the greenhouse gases in the earth’s atmosphere have risen from zero to 1, while in the next 30 years, till 2020, AGGI grew faster and hit 1.47 (AGGI 2022). In other words, nowadays, the earth’s atmosphere absorbs more energy than the pre-industrial days, around 3.18 W/m2, which leads to a faster change in the global climate (AGGI 2022). The increasing rate of greenhouse gas emissions has led to a drastic global warming, increasing sea levels, intense natural disasters, forests firing and, melting snows (Karimi et al. 2018b, 2020b, 2022b).
It is noteworthy that around 66% and 16% of that warming effect is related to carbon dioxide and methane, respectively (Karimi et al. 2018b, 2020b, 2022b). The recent data of different greenhouse gases contributions in climate warming are illustrated in Fig. 3.
Fig. 3.
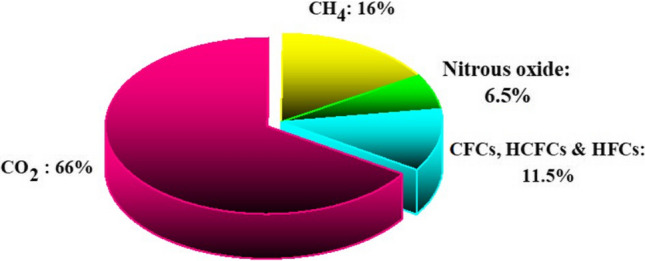
The share of different greenhouse gases in the global warming including carbon dioxide, methane, nitrous oxide as well as chlorofluorocarbon, hydrochlorofluorocarbon and hydrofluorocarbon (Dong et al. 2019). CO2, carbon dioxide; CH4, methane; CFC, chlorofluorocarbon; HCFC, hydrochlorofluorocarbon; HFC, hydrofluorocarbon
Because of all aforementioned challenges, currently, carbon dioxide with the most significant share in greenhouse gas emissions and global warming is in the center of attentions through scientific efforts. For instance, only coal firing and natural gas combustion were responsible for about 14.8 and 7.35 Gt global carbon dioxide emissions in 2021, respectively (IEA 2022). Regrettably, the carbon dioxide emission follows an increasing rate, which is now more than 40 Gt, annually (Karimi et al. 2020a), and as anticipated, the carbon dioxide emission reaches to around 46 Gt in 2030 (Dong et al. 2019).
It should be considered that the average concentration of carbon dioxide from pre-industrial days up to now has enhanced from 280 to 400 ppm (Karimi et al. 2018b; Regufe et al. 2021), which fossil fuel combustion is in charge of 75% of the emission (Karimi et al. 2020a). On the other hand, in different regions, carbon dioxide emissions have different growth trends through the years, in which, North America and Europe had the highest cumulative net anthropogenic carbon dioxide emission from 1850 till 2019 followed by Eastern Asia with 560, 380, and 300 Gt, respectively (IPCC 2022). All evidence prove that the carbon dioxide emission is significantly raising, and serious efforts are required to mitigate the greenhouse gas emissions for saving the planet and being in the way of net zero emission (Osman et al. 2022).
Biogas as a renewable source of energy
In the last decades, a massive attention has been devoted on developing renewable energy resources and specifically biogas production, to mitigate and overcome the global warming issue resulted from fossil fuel consumption (Shirzad et al. 2019b). Biogas is produced from degradation of the organic parts of wastes through the anaerobic digestion (Baena-Moreno et al. 2019). In such a way, heat, electricity, and fuel can be produced as well as the generated wastes are managed in a sustainable way (Shirzad et al. 2019b; Karimi et al. 2022b). Although, the benefits of biogas are already demonstrated and significant efforts have been made to supply the energy demands from bioenergy; nevertheless, a remarkable difference in global energy supply has not been observed since 2000 (WBA 2022).
Figure 4a represents a comparison between the global energy supply and the renewable sector increasing rate through the last two decades (WBA 2022). As can be observed, bioenergy production has increased at a slower rate than total energy production. In 2018, 31% of total energy supply was related to the oil sector. Also, nuclear, coal, gas, and renewable resources contributed to global energy supply with shares of 5%, 27%, 23%, and 14%, respectively (WBA 2022; Farghali et al. 2022). It is concluded that the global energy supply strictly depends on fossil fuels. It is worth mentioning that in 2018, only 11.3% of total energy supply was attributed to the bioenergy (WBA 2022).
Fig. 4.
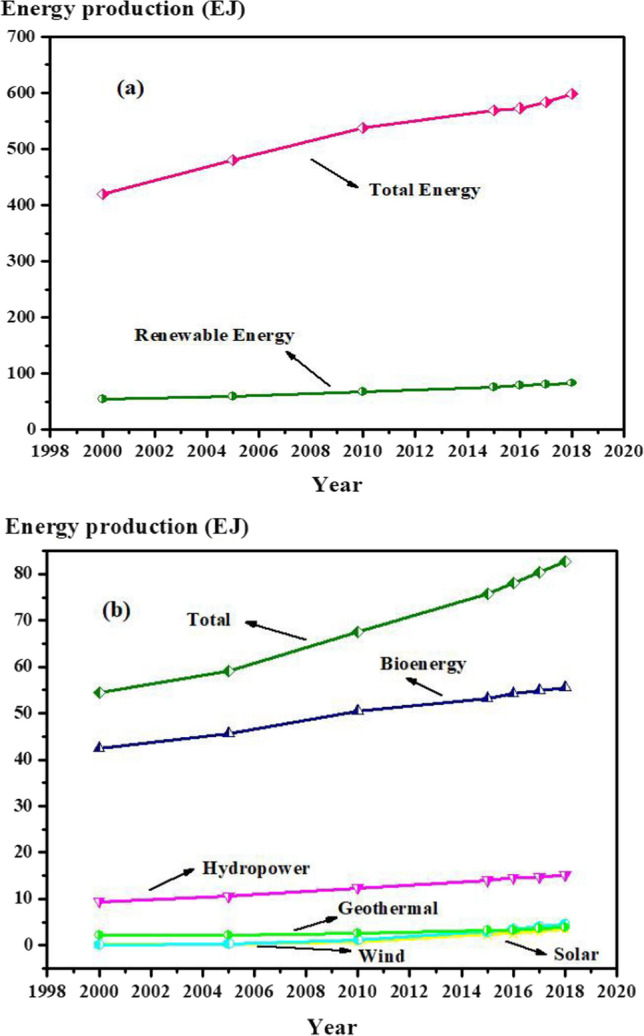
Comparison between a the total global energy supply and the total renewable sector increasing rate and b different produced renewable energies globally during the last two decades including hydropower, solar, wind, geothermal and bioenergy (WBA 2022)
Figure 4b illustrates a comparison between the total renewable energy supply and the shares of bioenergy, solar, wind, hydro, and geothermal sectors from 2000 to 2018 (WBA 2022). As shown, the major share of renewable energy is ascribed to the bioenergy, which is originated from difference sources such as the agricultural wastes, municipal solid wastes and, wastewater treatment sludge. As mentioned earlier, bioenergy is a reassuring global source of energy, particularly for European countries, which face with energy crisis.
In Europe, 75% of the total greenhouse gas emission is related to the energy sector. To this context, greenhouse gas emissions is planned to reach 55% and zero up to 2030 and 2050, respectively, by increasing the share of renewable energy sector in European Union’s overall energy consumption pattern (European Commission 2022a, b). It is also noteworthy that due to the recent war in Ukraine as well as Europe’s dependence on Russian fossil fuels, the European Commission established a new target for renewable energy generation by increasing the former target agreed by European Union leaders in 2014 from 27 to 45%, according to European Commission’s plan (REPowerEU) in 2022 for renewable energy share in 2030.
In other words, Europe’s renewable energy generation must reach to 1067 GW before 2030 (European Commission 2022a, b). In this context, European countries require to develop new plants to produce more energy from renewable sources. On the grounds, biogas production through the sustainable deployment can play a key role and contribute to a considerable reduction in the greenhouse gas emissions. To date, European countries require a comprehensive action plan to achieve the demand by increasing the capacity using diverse sustainable processes. It is estimated that 35 billion cubic meters of biomethane are demanded to meet the defined target before 2030 (Sacha Alberici et al. 2022). In 2022, Guidehouse Netherlands B.V. estimated the potential of European Union countries biomethane production by anaerobic digestion in 2030 to meet the European Commission target, which is depicted in Fig. 5 (Sacha Alberici et al. 2022).
Fig. 5.
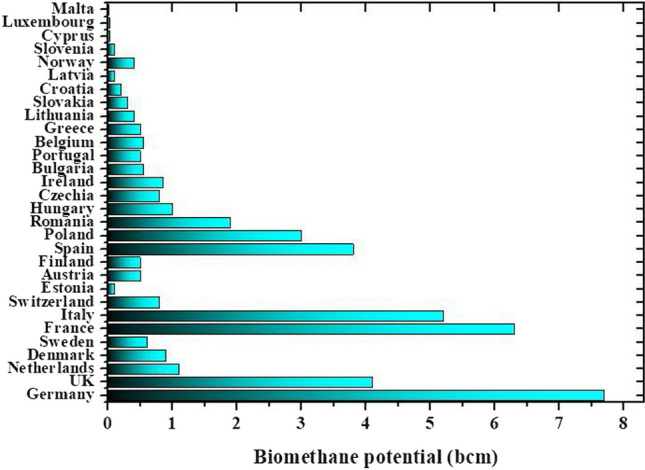
Estimation of Guidehouse Netherlands B.V. for the European Union plus UK countries biomethane production potential by anaerobic digestion in 2030 to meet the European Commission target (Sacha Alberici et al. 2022). bcm, billion cubic meters
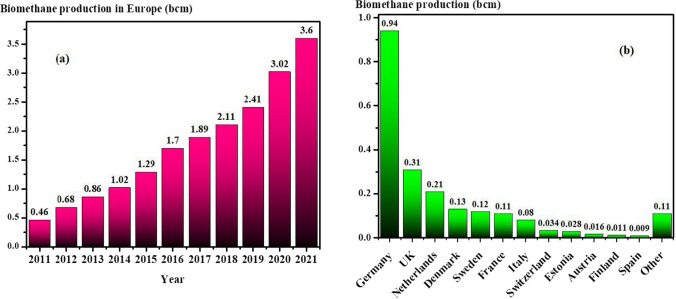
The state-of-the-art biomethane production in Europe is demonstrated in Fig. 6a, which clearly approves that the biomethane production capacity is dramatically lower than the European commission target for 2030 (European Commission 2022b; Sacha Alberici et al. 2022). Figure 6b indicates the European countries biomethane production separately in 2018 (EBA 2021), which we can conclude that many countries require to improve their roadmaps to meet the defined targets. To this end, scientific and financial supports as well as, economic motivations are required. It is notable that according to International Energy Agency (IEA) report in 2020, the average of biomethane production price is 19 $/MBtu (EBA 2021; European Commission 2022b). However, after the Ukraine crisis, the gas price in Europe reached up to 50 $/MBtu, which is significantly higher than biomethane (EBA 2021; European Commission 2022b).
Fig. 6.
a Biomethane production trend in European countries from 2011 to 2021 (European Commission 2022b; Sacha Alberici et al. 2022). b The capacity of biomethane production of each European country countries in 2018 (EBA 2021). bcm, billion cubic meters
As indicated in Table 1, the biogas compositions strongly rely on the feed type and the process of biogas production. For instance, the produced biogas by the anaerobic digestion process is contained approximately 40% of carbon dioxide (Karimi et al. 2022b). Accordingly, the biogas to meet the pipelines standards as a domestic fuel and/or ship for other industrial applications requires to be upgraded by separating the carbon dioxide and other contaminants from methane (Muñoz et al. 2015).
Table 1.
Comparison of biogas characteristics originated from anaerobic digestion and landfill, as well as the biomethane standards for pipeline (Spitoni et al. 2019; Naquash et al. 2022)
| Parameter | Biogas from anaerobic digestion | Biogas from landfill |
|---|---|---|
| Minimum methane number | 135 | 130 |
| Lower heating value (MJ/kg) | 23 | 16 |
| Wobbe index (MJ/Nm3) | 27 | 18 |
| Density (kg/Nm3) | 1.2 | 1.3 |
| Composition (%mol) | ||
| CH4 | 60–70 | 35–65 |
| CO2 | 30–40 | 15–40 |
| H2O | 1–5 | 1–5 |
| N2 | 0.2 | 15 |
| H2 | 0 | 0–3 |
| Heavy carbon | 0 | 0 |
| Composition (ppm) | ||
| Ammonia | Less than 100 | 5 |
| H2S | 0–10,000 | 0–100 |
| Standards for biomethane in gas and liquid form | ||
|---|---|---|
| Components | Composition (upper limit) | |
| Gas form | Liquid form | |
| H2O | 32 (mg/m3) | 1 ppmv |
| H2S | 5 (mg/m3) | 4 ppmv |
| CO2 | 3% | 25 ppmv |
CO2, carbon dioxide; CH4, methane; H2O, water; N2, nitrogen; H2, hydrogen; H2S, hydrogen sulfide
Carbon dioxide capture techniques
Generally, carbon dioxide mitigation methodologies are categorized in three main ones including post-combustion, pre-combustion, and oxy-fuel combustion (Karimi et al. 2021c), which are described in following.
Post-combustion carbon dioxide capture
Post-combustion carbon dioxide capture is ascribed to the process of carbon dioxide sequestration from the flue gases after combustion process and before emission to atmosphere. In these processes, the flue gas passes through a carbon dioxide removal unit and hence, a carbon dioxide-free flue gas is emitted to the atmosphere. One of the most important obstacles through the post-combustion processes is the low partial pressure of carbon dioxide, normally from 3 up to 15% v/v in the flue gas (Riboldi 2016; Karimi et al. 2021c). Other barriers in the post-combustion processes can be mentioned as the huge volume of flue gas, which is introduced to carbon dioxide removal unit and the presence of impurities, which can adversely affect the performance of carbon dioxide capture process (Riboldi 2016; Karimi et al. 2021c).
Pre-combustion carbon dioxide capture
Routinely, this process is related to the carbon dioxide separation from biogas, natural gas, and syngas to be considered as a clean fuel to meet the standards of pipelines or considering for combustion process. In this way, the biogas is upgraded, and natural gas is sweetened, also through the syngas process, the produced carbon monoxide and hydrogen undergo a further reaction, shift reaction, by means of steam to generate more hydrogen (Riboldi 2016; Osman et al. 2020).
Further, carbon dioxide removal step is required to produce an enriched hydrogen stream as the combustion fuel feed. In pre-combustion processes, the carbon dioxide volumetric composition is typically between 15 and 60% with the total pressure of 2–7 MPa. To this context, carbon dioxide removal in the pre-combustion is companied with less energy consumption demand (Riboldi 2016; Osman et al. 2020).
Oxy-fuel combustion carbon dioxide capture
Oxy-fuel combustion carbon dioxide capture is assigned to the process of carbon dioxide separation from the feed oxidization with pure oxygen (Karimi et al. 2021c). The high-purity carbon dioxide is produced in this process owing to the pure oxygen utilization, instead of air. The technology of carbon dioxide separation in this process is equivalent to the post-combustion carbon capture process. Due to the high purity of carbon dioxide, the separation process is accomplished with much higher efficiency (Osman et al. 2020).
It is worth mentioning that in this process, the combustion temperature reaches up to 3500 °C (Riboldi 2016), which is detrimental to the combustion equipment. In-line with that the combustion temperature shall be decreased to around 1400 °C and 1900 °C for the typical gas turbine and oxy-fuel coal-fired turbines, respectively. To this end, a recycle flue gas is inserted to the combustion chamber and moderate the combustion temperature (Riboldi 2016; Osman et al. 2020). A comparison concerning the benefits and drawbacks of each category is illustrated in Table 2.
Table 2.
Benefits and drawbacks of carbon dioxide capture processes (Younas et al. 2020; Osman et al. 2020; Karimi et al. 2021c)
| Category | Benefits | Drawbacks |
|---|---|---|
| Post-combustion | Maturity comparing with available carbon capture processes | High energy consumption |
| Facility of retrofitting the technology to the new and existing plants | Low concentration of CO2 in flue gas which reduces the efficiency of process | |
| The presence of various impurities | ||
| Requires adsorbent with high CO2 uptake capacity and high selectivity | ||
| Pre-combustion | High CO2 concentration in syngas and biogas enhances the efficiency of the process | High capital cost |
| Fully developing for wide-scale adoption and commercialization | Decay challenges regarding the hydrogen-rich fuel consumption | |
| Low cost of adaptation | The presence of impurities such as H2S and CO | |
| Heat transfer problems | ||
| Oxy-fuel combustion | Relatively simple technology | High capital and operating cost |
| Suitable for retrofit | High energy consumption | |
| NOx is not significant | Requires large quantities of oxygen |
CO2, carbon dioxide; CO, carbon monoxide; H2S, hydrogen sulfide; and NOx, nitrogen oxides
Carbon dioxide separation technologies
According to the process nature, carbon dioxide separation/capture technologies can be classified in five major classes including membrane, absorption, adsorption, cryogenic distillation, and chemical looping (Thomas and Benson 2005). Carbon dioxide capture by membrane technology includes a barrier between two different phases, which leads the carbon dioxide separation from the next components (Shirzad et al. 2019a). Generally, three types of membranes have been applied in carbon dioxide separation applications such as 1-polymeric membranes, 2-inorganic membranes, and 3-mixed matrix membranes (Yang et al. 2008).
Carbon dioxide absorption process as the most prevalent technology is defined as dissolving carbon dioxide in a media, through the physical or chemical phenomena (Yu et al. 2012). Also, carbon dioxide capture through the adsorption process is implemented using an adsorbent, physically or chemically (Roque-Malherbe 2018). Further, cryogenic carbon dioxide separation is a technology implemented in high pressure and very low temperature, in which carbon dioxide is separated according to different boiling points (Xu et al. 2012).
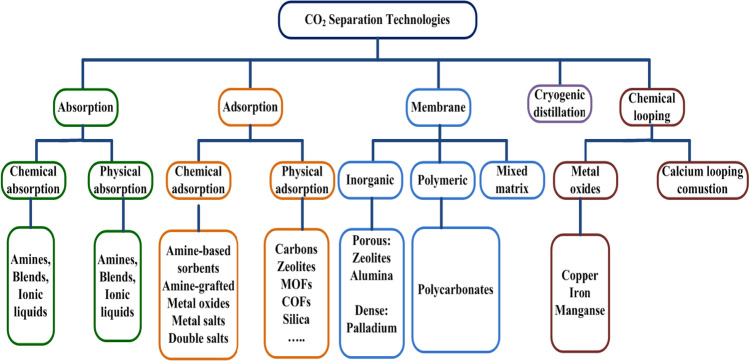
Different carbon dioxide separation/capture processes are schematically represented in Fig. 7, accompanied by some key instances for each technology. On the other hand, energy consumption and cost including operating, and maintenance are two major factors toward the appropriate separation technology for carbon capture and storage and biogas upgrading, which these different technologies have been compared concerning these factors in Fig. 8 (Spitoni et al. 2019).
Fig. 7.
Technologies for carbon dioxide separation and capture including absorption, adsorption, membrane, cryogenic and chemical looping (Canevesi et al. 2018; Madejski et al. 2022). CO2, carbon dioxide; MOF, metal–organic framework; COF, covalent organic framework
Fig. 8.
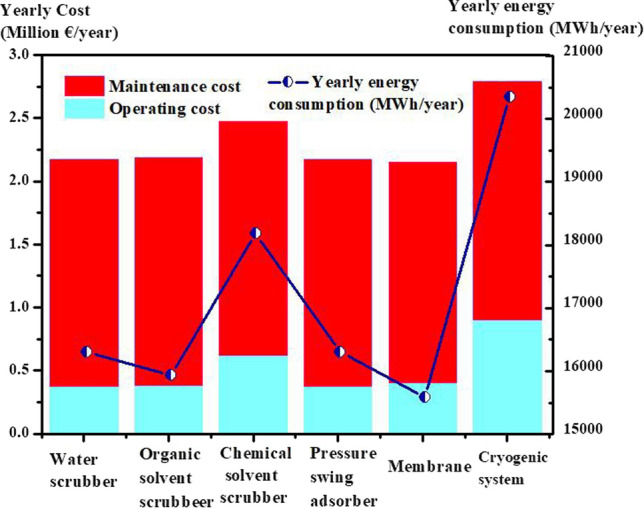
Comparison between energy consumption, maintenance cost and operating cost of different carbon dioxide separation and capture technologies such as water scrubber, organic solvent scrubber, chemical solvent scrubber, pressure swing adsorption, membrane and cryogenic (Spitoni et al. 2019). MWh, megawatt-hour
Further, specific capital cost, advantages and limitations of these techniques have also been specified in detail in Tables 3 and 4 (Leung et al. 2014; Canevesi et al. 2018; Naquash et al. 2022). As can be found, the high cost, environmental issues, and high energy consumption have faced the applications of membrane, cryogenic, and absorption technologies with serious challenges. On the other hand, adsorption technology not only is an eco-friendly technology, but also represents a low-energy consumption process, which is able to selectively separate carbon dioxide in an efficient way. Accordingly, recently it has received a significant attention regarding its applications for carbon capture and storage as well as biogas upgrading (Ho et al. 2008; Khajeh and Ghaemi 2020; Jin et al. 2021; Karimi et al. 2022b).
Table 3.
Capital cost of carbon dioxide separation technologies (Naquash et al. 2022)
| Separation technology | Specific capital cost (€/(Nm3/h)) |
|---|---|
| Water scrubber | 5500 |
| Organic solvent scrubber | 4500 |
| Chemical solvent scrubber | 3200 |
| Pressure swing adsorber | 2700 |
| Membrane | 2500 |
| Cryogenic system | 5600 |
Nm3/h, normal cubic meters per hour
Table 4.
Advantages and limitations of carbon dioxide separation/capture technologies (Clausse et al. 2011; Leung et al. 2014; Canevesi et al. 2018)
| Technology | Advantages | Limitations | Purity and Recovery |
|---|---|---|---|
| Adsorption | Reusable adsorbents | In some cases, requires pretreatment for separating other impurities including H2S and water vapor | PSA, Purity: 50–99% & Recovery: 30–90% |
| High efficiency | Requires adsorbents with very specific characters | TSA, Purity: 95% & Recovery: 80% | |
| Mature technology | High energy consumption in terms of flue gas and TSA process | ESA, Purity: 20% & Recovery: 93% | |
| Environmentally friendly | |||
| Quick and simple installation | |||
| Chemical absorption (with a reagent) | High efficiency | Highly energy consumption | |
| The most matured technology | Corrosion effects | ||
| Environment problems | Purity: 99% & Recovery: 98% | ||
| Absorption highly depends on CO2 concentration | |||
| Physical absorption (with water) | High purity | Purified gas requires a further drying step | |
| H2S removal also occurs | Energy requirement for water cooling | Purity: 99% & Recovery: 98% | |
| Matured technology | Environment problems | ||
| Cryogenic | Large-scale applications are already available | Highly energy consumption | |
| Matured technology | Only viable for high CO2 concentration | Purity: 99.9% & Recovery: 90% | |
| Large equipment requirement | |||
| Membrane | High purity | Low capacity | |
| Low energy requirement | Fouling | ||
| Simple installation | Low selectivity | Purity: higher than 95% & Recovery: 90% | |
| Compact structure | High cost | ||
| Well knowledge on technology | H2S removal requirement before CO2 adsorption |
PSA, pressure swing adsorption; TSA, temperature swing adsorption; ESA, electric swing adsorption; H2S, hydrogen sulfide; and CO2, carbon dioxide
Lately, many efforts have been conducted for optimizing the carbon dioxide adsorption process, reducing the energy consumption, consequently decreasing the operating cost, as well as developing the sustainable sorbents for carbon capture, which detailed description of these investigations have been extensively discussed in the following sections (Ho et al. 2008; Khajeh and Ghaemi 2020; Jin et al. 2021; Karimi et al. 2022b).
Recent adsorbents for carbon dioxide capture
Through the evolution of adsorbents, various types of samples including zeolites, activated carbons, metal–organic frameworks, covalent organic frameworks, and silicates have been developed for carbon capture and sequestration (Lai et al. 2021). Generally, carbon dioxide adsorbents are categorized in two different classes such as physical adsorbents, and chemical adsorbents (Lai et al. 2021). In physical adsorbents, the textural properties including size, surface, and design of pores play as the key elements on the adsorption performance. As a basic rule, larger volume/surface area with the pore diameter less than 0.7 nm is more favorable for adsorption processes (Presser et al. 2011; Kumar et al. 2020).
Furthermore, an optimum value for enthalpy of sorption, which satisfies the adsorption and desorption quality is in-line with the carbon dioxide capture purposes (Presser et al. 2011; Kumar et al. 2020). On the other hand, the chemical adsorbents are commonly metallic elements, which have been employed in either salt or oxide forms including magnesia and calcium oxide, alkali-metal compounds as lithium silicate, hydrotalcites, lithium zirconate, and double salts (Songolzadeh et al. 2012). In this way, during the adsorption process the acidic gas, such as carbon dioxide reacts with adsorbent to create metal carbonates in the strong chemical bonds. Accordingly, the desorption of chemical adsorbents as highly energy waster is the main drawback of this class of sorbents (Ben-Mansour et al. 2016).
However, carbon dioxide capture at the high temperature, over than 300 °C, is also considered as the main benefit of these sorbents. It is notable that in some cases to improve the reversibility of chemical adsorbents, chemical treatments using alumina with alkali-metal carbonates and/or alkaline metal oxides are employed (Yong et al. 2002).
The carbon dioxide adsorbents in another classification are also categorized based on the adsorption operation temperature in three different classes including low-temperature carbon dioxide adsorbents for operation temperature less than 200 °C, intermediate-temperature carbon dioxide adsorbents for operation temperature between 200 and 400 °C, and also high-temperature carbon dioxide adsorbents for operation temperature higher than 400 °C (Wang et al. 2010).
An adsorbent to be considered as a proper candidate for carbon capture and storage as well as biogas upgrading applications shall represent some key specifications, which the basic ones are high surface area, high porosity, minimum thermal degradation, chemical stability, high carbon dioxide selectivity, low cost of synthesis and proper loading capacity (Akinola et al. 2022). In the following, different classes of carbon dioxide adsorbents have been discussed in detail for such purposes concerning these phenomena.
Zeolites
Zeolite was firstly introduced in 1756, by the Axel Fredrik Cronstedt (Ruthven 1984), which because of their significant positive specifications, study on zeolites rapidly grew and today, they are a major element of adsorption technology (Choi et al. 2009; Kumar et al. 2020). Totally, zeolites are silicate-based materials composed with alumina to result a crystalline structure, high surface area, and tunable pore design (Kumar et al. 2020). On the ground, various parameters may affect the zeolite adsorption capability such as alumina content in zeolite structure and the adsorbent pore diameter (Barthomeuf 2002; Henrique et al. 2019). In fact, the alumina content determines the total exchangeable cations concentration in the adsorbent structure (Barthomeuf 2002).
On the other hand, the studies on some types of zeolites approved the one with larger pore diameter, zeolite 13X showed higher adsorption capability than other types of zeolites including 4A, 5A, APG-II, and WE-G 592 (Siriwardane et al. 2005). Further, employing the alkali and alkaline earth metals demonstrated positive impacts on the zeolite physical properties and consequently, their adsorption capacity (Shen et al. 2002). Structurally, zeolites differ in Si/Al ratio, the charge balancing cation type, and operationally, the pretreatment process procedure (Byung Yoon 2002).
It is worth mentioning that the effect of earth metal modifier addition to the zeolites on their long-term stability is still unknown (Choi et al. 2009). For instance, Table 5 indicates some key physical and chemical properties of prevalent zeolites 13X, 5A, 4A, WE-G 592, and APG-II (Siriwardane et al. 2005; Nikolaidis et al. 2018; Zhao et al. 2018).
Table 5.
Zeolite’s physical and chemical properties (Siriwardane et al. 2005; Nikolaidis et al. 2018; Zhao et al. 2018). CO2, carbon dioxide and N2, nitrogen
| Property | Unit | Type of zeolitesa | ||||
|---|---|---|---|---|---|---|
| 4A | 5A | APG-II | WE-G 592 | 13X | ||
| Earth metal modifier | – | NAb | Ca | NA | NA | NA |
| Pore diameter | Å | 4 | 5 | 10 | 10 | 10 |
| Zeolite surface area | m2.g−1 | NA | NA | 710 | 625 | 710 |
| Aluminum content | wt% | 13.6 | 14.8 | 10.7 | 15.6 | 14.2 |
| Silicon content | wt% | 16.1 | 16.7 | 14.3 | 16.5 | 18.2 |
| Breakthrough timec | min | 9 | 13 | 15 | 24 | 20 |
| Adsorbent mass | g | 7.21 | 6.53 | 6.40 | 12.46 | 19.3 |
| CO2 loadingc | mmol g−1 | 0.5 | 0.38 | 0.38 | 0.6 | 0.7 |
| Gas flow rate | cm3 min−1 | 5 | 5 | 5 | 5 | 5 |
| selectivity (CO2/N2)c | – | NA | NA | NA | 15.5 | 23.11 |
| Potential application | – | NA | NA | NA | Post-combustion | Post-combustion |
| Heat of adsorptionc | kJ mol−1 | − 56 | − 63 | − 66 | − 55 | − 63 |
aAll zeolites are represented by their commercial names
bNot available
cData reported at 120 °C and 1 atm
Besides the promising features of zeolites for carbon dioxide adsorption, however some drawbacks still constrain the zeolite applications. In general, at the higher temperatures, carbon dioxide adsorption on zeolites significantly reduces and tends to zero at 200 °C (Inui et al. 2002). The low selectivity of zeolites is another drawback of available zeolites for carbon capture and sequestration as well as biogas upgrading purposes (Kumar et al. 2020). Additionally, zeolites represent high hydrophilic character, which significantly decreases their performance at the humid operating conditions, especially their regeneration (Choi et al. 2009).
Carbon-based adsorbents
Activated carbon
Activated carbon is a substance addressing the carbon-rich materials, which includes well-structured porosity and carbon networks. A wide range of chemical functional groups on the surface of these samples, highly organized macro-, meso-, and micro-pores, accompanied with high surface area nominate this sample in broad range of applications (Karimi et al. 2021a). Accordingly, recently it has been considered as a versatile adsorbent to be employed for wastewater treatment, pharmaceutical science, air pollution control from inhabited places, automobile pollution control devices, solvent recovery, food processing industry, and gas mask filter manufacturing technology (Karimi et al. 2021a).
Alongside of all mentioned applications, one of the main domains of activated carbon application is being an adsorbent for gas adsorption (Karimi et al. 2021a, 2022b). On the grounds, recently numerous studies have been devoted to the engineering aspect and/or material nature of activated carbon for carbon dioxide capture (Karimi et al. 2022b). Routinely, carbon-based adsorbents regarding their hydrophobic character, fast kinetic, large surface area, tunable structure, high chemical and thermal stability, low heat of adsorption, large diversity of sources, and reasonable efficiency are among the oldest and favorable adsorbents for carbon capture studies (Karimi et al. 2018b, 2020a, b).
Despite of several benefits of activated carbons, the low selectivity is one of the major drawbacks of this adsorbent for gas adsorption concerning the separation of carbon dioxide and methane and/or carbon dioxide and nitrogen, especially at low percentage of carbon dioxide. Accordingly, in the recent years, a major attention has been devoted to the modification of activated carbons, which activating reagents, temperature, and reagent/carbon mass ratio are considered as the most effective parameters on the modifications/activations of commercial activated carbon as carbon dioxide adsorbent (Sreńscek-Nazzal and Kiełbasa 2019).
Through the years, researchers tried to improve the textural properties of commercial activated carbons by introducing new media to the activated carbons structures (Sreńscek-Nazzal and Kiełbasa 2019), impregnation via chemical reagents (Liu et al. 2013b), or through the dielectric barrier discharge plasma (Glonek et al. 2017). In this way, recently Karimi et al. (Karimi et al. 2018b) employed different chemical treatments using hydrogen peroxide, sulfuric acid, nitric acid, and urea as well as thermally modifications till 800 °C to improve the characters of commercial activated carbons.
Further, Srenscek-Nazzal et al. (2016) modified the commercial activated carbon by potassium hydroxide, potassium carbonate, and zinc chloride and reported an enhancement on carbon dioxide adsorption capacity due to the optimized structural properties. Some of helpful information concerning the surface modification and activation of carbon materials can be found in (Abd et al. 2021).
Biomass/biochar-originated carbon dioxide adsorbent
Lately, novel activated carbons derived from renewable and plentiful resources, which are abundant and economic, such as agricultural wastes, food wastes, animal manure, and industrial by-products, have attracted massive interests for carbon dioxide adsorption applications (Karimi et al. 2022b). Annually, thousands of tons of these wastes are dumped or burnt throughout the world, without any beneficial usage that leads to drastic environmental concerns (Patra et al. 2021). For instance, Table 6 indicates the potential of some biomass and animal wastes throughout the world, which have already been evaluated as a source of adsorbent (Karimi et al. 2022b).
Table 6.
Biomass production in various countries
| Biomass/waste | Production (M tons/y) | Country | Year | References |
|---|---|---|---|---|
| Soybean | 41.65 | Argentina | 2018 | FAO (2022) |
| Rapeseed | 21.48 | Canada | 2020 | OurWorldinData (2022) |
| Bagasse | 758 | Brazil | 2020 | Silalertruksa and Gheewala (2020) |
| Wood pellet | 52.7 | Worldwide | 2020 | Saosee et al. (2020) |
| Wheat | 281.15 | Europe | 2020 | OurWorldinData (2022) |
| Corncob | 20 | China | 2015 | Li et al. (2015b) |
| Pine sawdust | 0.28 | Mexico | 2019 | Parascanu et al. (2019) |
| Cassava | 66.14 | Nigeria | 2020 | OurWorldinData (2022) |
| Sweet potato | 7.62 | Malawi | 2020 | OurWorldinData (2022) |
| Soybean | 134.26 | Brazil | 2020 | OurWorldinData (2022) |
| Pea | 3.02 | Russia | 2020 | OurWorldinData (2022) |
| Sugar crops | 408.41 | India | 2020 | OurWorldinData (2022) |
| Potato | 24.8 | Ukraine | 2018 | FAO (2022) |
| Rice (paddy) | 91.54 | Indonesia | 2018 | FAO (2022) |
The biomass usually undergoes a conversion process, incomplete combustion such as pyrolysis, to produce bio-oil as well as carbon dioxide and digestate. In this way, the digestate, through an aerobic process is transformed to biochar (Karimi et al. 2021a), which as a low-cost and enriched carbon precursor for carbon dioxide adsorption is promising not only in the view of abundant resources, but also as a prominent strategy for solid wastes management and carbon dioxide capture in a sustainable way to be substituted with commercial adsorbents (Karimi et al. 2022b).
In-line with that numerous studies have investigated the feasibility of various carbon precursors including olive, pine sawdust, oat, wood pellet, coconut, almond, oil tea, whitewood, rice, peanut, palm, coconut shell, pinecones, wood ash, bagasse, corncob, argan fruit shells, eggshell, animal wastes, and bio-sludge of waste water treatment plant as a source of adsorbent for carbon capture (Karimi et al. 2022b). Further, researchers proved that the huge volume of municipal solid wastes, which adversely affect the planet and the human life, can be sustainably managed by turning these materials to energy and also the activated carbon, through the anaerobic digestion and afterward, an activation process (Karimi et al. 2021c; Madejski et al. 2022).
Commonly, to produce activated carbon from carbonaceous resources, after carbonization, a pretreatment process is required (Sharma et al. 2021). In this context, the carbonized samples are washed and then dried at about 100 °C (Karimi et al. 2020a, 2022b). Afterward, the fully dried samples are grinded to be prepared for the activation step (Karimi et al. 2020a, 2022b). Two routine methods for sample activation are physical and chemical treatments. The physical activation consists of a two-step procedure, in which the sample is firstly carbonized at temperature between 400 and 850 °C, afterward, activated by steam, carbon dioxide and air (Karimi et al. 2018b, 2020a, 2022b).
On the other hand, chemical activation includes a one-step activation method, implemented by means of some reagents such as hydrochloric acid, phosphoric acid, sulfuric acid, and sodium hydroxide. It is worth mentioning that in some cases, two activating methods are utilized simultaneously, either physical at the first or chemical (Karimi et al. 2020b). It should be considered that various parameters affect the biochar-to-adsorbent process such as conversion temperature, heating rate through the conversion, feed’s nature, chemical reagent, and conversion process duration. Accordingly, pyrolysis, gasification, torrefaction, and hydrothermal carbonization are the main conversion processes of biomass to biochar mentioned in the literature (Shirzad et al. 2019b).
A comparison between some prevalent precursors activated through physical or chemical process and other carbonaceous-based adsorbents for carbon dioxide capture is reported in Table 7. It should be considered that moderate heat of adsorption of carbon dioxide on derived activated carbons from biomass/biochar leads to a convenient regeneration process. In this way, manifold low-cost biomass/biochar-originated activated carbons have been investigated under cyclic adsorption processes (Karimi et al. 2022b). Nowadays, activated carbons from spent materials, which showed reasonable carbon dioxide uptake and high regenerability, can play a significant role in carbon dioxide sequestration and global warming mitigation through a fully sustainable path (Karimi et al. 2020b).
Table 7.
Comparison of biomass precursors activated through physical or chemical process and other carbonaceous-based adsorbents for carbon dioxide capture
| Samples | Activation method | Operational condition | CO2 capacity (mmol/g) | Selectivity | Potential application | References | |
|---|---|---|---|---|---|---|---|
| Temperature (◦C) | Pressure (bar) | ||||||
| Rice husk ash | Chemical | 50 | 1 | 2.95 | – | – | Hemalatha et al. (2012) |
| Date seed | Chemical | 20 | 1 | 1.79 | – | – | Ogungbenro et al. (2020) |
| Sunflower seed shell | Chemical | 25 | 1 | 4.55 | CO2/N2: 6.69 | Post-combustion | Deng et al. (2015) |
| Beer waste | Physical | 0 | 1 | 2.6 | CO2/N2: 13 | Post-combustion | Hao et al. (2013) |
| Oil tea shell | Chemical | 0 | 1 | 6.15 | CO2/CH4: 2.37 | Biogas upgrading | Zhang et al. (2018) |
| Lignin waste | Chemical | 0 | 1 | 7.4 | – | – | Sangchoom and Mokaya (2015) |
| Peanut shell | Chemical | 0 | 1 | 7.45 | CO2/N2: 8.28 | Post-combustion | Li et al. (2015a) |
| Rambutan peel | Physical | 30 | 1 | 1.70 | CO2/N2: 30 & CO2/CH4: 37.5 | Post-combustion & biogas upgrading | Zubbri et al. (2020) |
| Peanut shell | Chemical | 0 | 1 | 4.0 | CO2/N2: 6.45 | Post-combustion | Deng et al. (2015) |
| Coffee ground | Physical/chemical | 0 | 1 | 4.9 | – | – | Plaza et al. (2012) |
| Olive stone | Physical | 0 | 0.95 | 4.65 | CO2/N2: 6.74 | Post-combustion | González et al. (2013) |
| Olive and cherry stone | Physical | 25 | 3 | 2.53 | CO2/CH4: 3.73 | Biogas upgrading | Álvarez-Gutiérrez et al. (2014) |
| Poplar anthers | Chemical | 25 | 1 | 4.15 | CO2/N2: 10.38 | Post-combustion | Song et al. (2014) |
| Horse manure | Physical | 0 | 1 | 3.8 | CO2/N2: 7.6 | Post-combustion | Hao et al. (2013) |
| Algae | Chemical | 25 | 1 | 4.5 | CO2/N2: 9 | Post-combustion | Sevilla et al. (2012) |
| Coconut shell | Physical | 30 | 1 | 2.45 | CO2/N2: 6.13 | Post-combustion | Wawrzyńczak et al. (2019) |
| Palm kernel | Physical | 30 | 4 | 7.32 | – | – | Nasri et al. (2014) |
| Tobacco wastes | Chemical | 25 | 1 | 2.6 | CO2/N2: 10.4 | Post-combustion | Sha et al. (2015) |
| Agaricus | Chemical | 0 | 1 | 5.5 | CO2/N2: 27.3 | Post-combustion | Wang et al. (2012) |
| Rice husk ash | Physical | 75 | 1 | 3.93 | – | – | Zeng and Bai (2014) |
| Beech wood | Physical | 23 | 1 | 2.54 | – | – | Gebald et al. (2014) |
| Municipal solid waste | Chemical/physical | 40 | 2.5 | 2.6 | – | – | Karimi et al. (2020b) |
| Sawdust, cellulose, and starch | Chemical | 25 | 1 | 4.8 | CO2/N2: 5.4 | Post-combustion | Sevilla and Fuertes (2011) |
| Commercial activated carbon | Chemical | 30 | 1 | 2.2 | CO2/N2: 11 | Post-combustion | Chen et al. (2021) |
| Commercial activated carbon | Physical | 25 | 1 | 1.88 | – | – | Rashidi and Yusup (2021) |
| Molecular sieve | Physical | 30 | 0.4 | 0.9 | – | – | al Mesfer et al. (2020) |
| Molecular sieve | – | 0 | 1 | 4.92 | CO2/N2: 11 | Post-combustion | Yang et al. (2020) |
CO2, carbon dioxide; N2, nitrogen; and CH4, methane
Carbon molecular sieve and graphene
Carbon molecular sieves belong to a special branch of carbon-based materials. These microporous structures possess pore diameter about 0.3 nm, which is tunable for different species separation (Lai et al. 2021). It is worth mentioning that the selectivity of carbon molecular sieves can be adjusted for specified adsorbate that is considered as a significant advantage in adsorption purposes. On the other hand, this type of adsorbent suffers from the high toxicity, high cost, and low carbon dioxide capacity (Lai et al. 2021).
Graphene, which has also been evaluated in some studies for carbon capture and sequestration (Huang et al. 2018; An et al. 2019; Zhou et al. 2021), is a two-dimensional carbonaceous material constructed in hexagonal-shape structures (Lai et al. 2021). The tunable layer characteristics and wide range of functionalization reagents through a matured technology specified graphene as a carbon dioxide adsorbent with high potential. However, sensitivity to the structural defects has hindered to extend its applications in carbon dioxide removal applications (Lai et al. 2021).
Metal–organic frameworks
Li et al. (1999) firstly introduced metal–organic frameworks as an appealing candidate for gas separation and storage, catalysis, and molecular recognition, which showed stable porosity without guest ions or solvent. They reported that these materials illustrate surface tuning and pore functionalization more than other conventional adsorbents (Li et al. 1999). Metal–organic frameworks are basically constituted of metal ions and clusters, and organic ligands with surface area and pore size designed up to 10,000 m2/g and 150 nm, respectively (Huang et al. 2003; Furukawa et al. 2013).
Totally, metal–organic frameworks are synthesized through various methods including solvothermal as most-repeated in the literature (Chen et al. 2017; Hsieh et al. 2022), electrochemical (Stassen et al. 2015), hydrothermal (Chen et al. 2019), microwave-assisted (Vakili et al. 2018), mechanochemical (Chen et al. 2018b), sonochemical (Vaitsis et al. 2022), template (He et al. 2019b), ionothermal (Zunita et al. 2022), atomic layer deposition (Lemaire et al. 2017), sol–gel (Shamsudin et al. 2019), ultrasound-assisted (Sargazi et al. 2017), spray-drying (Mitsuka et al. 2021), and flow chemistry (He et al. 2019a), which is the most up-to-date one (Rubio-Martinez et al. 2017).
However, almost the synthesis procedures of all metal–organic frameworks pursue a similar protocol, but the final structural form possesses a major effect on adsorption capacity (Hiyoshi et al. 2005; Serna-Guerrero et al. 2008). The limitations of some of the most-employed metal–organic frameworks synthesis techniques are described in Table 8 (Stassen et al. 2015; Sargazi et al. 2017; Rubio-Martinez et al. 2017; Vakili et al. 2018; Yulia et al. 2019).
Table 8.
Limitations of the most-employed metal–organic frameworks synthesis methods. Zr, zirconium; MOFs, metal–organic frameworks
| MOF synthesis method | Limitations | Reference |
|---|---|---|
| Solvothermal | High energy consumption | Sargazi et al. (2017) |
| Slow reaction | ||
| Electrochemical | Attributed to Zr-based MOFs | Stassen et al. (2015) |
| Acid consumption | ||
| Hydrothermal | Slow reaction | Chen et al. (2019) |
| Sonochemical | Rarely applied | Ghanbari et al. (2020) |
| Not accommodation with organic solvent | ||
| Microwave-assisted | Nonthermal effects | Vakili et al. (2018) |
| Ultrasound-assisted | Cost issues | Sargazi et al. (2017) |
| Mechanochemical | Slow reaction | Sargazi et al. 2017; Yulia et al. (2019) |
| High temperature | ||
| High pressure | ||
| Large production limitations | ||
| Flow chemistry | Unmatured | Rubio-Martinez et al. (2017) |
| Spray-drying | Process difficulties | Rubio-Martinez et al. (2017) |
The adsorption kinetics of numerous metal–organic frameworks follow the Langmuir-like form, in which, at the low carbon dioxide partial pressure, a small change in the pressure leads to a large differentiation in carbon dioxide adsorption capacity. On the other hand, there is an induction gap in the adsorption isotherm of several metal–organic frameworks. To this context, in moderate pressure, a slight change in pressure contributes to a significant variation in adsorption capacity; on the other hand, in lower pressure, the capacity variation would be negligible (Choi et al. 2009).
As mentioned earlier, various metal–organic frameworks exhibit low carbon dioxide capacity in lower pressure range of around 0.1–0.2 bar. It is noteworthy that the intermolecular forces between adsorbed carbon dioxide and adsorbent are typically insignificant. Hence, adsorbent regeneration in the cyclic adsorption processes occurs in low temperatures, around 30 °C (Wang et al. 2010). A comparison between carbon dioxide uptake capacities of some of the most popular metal–organic frameworks is demonstrated in Table 9.
Table 9.
Comparison of recently developed metal–organic frameworks for carbon dioxide capture and sequestration
| MOFsa | BET surface area (m2/g) | Pressure (atm) | Temperature (°C) | CO2 uptake (mmol g−1) | Selectivity | Potential application | Reference |
|---|---|---|---|---|---|---|---|
| Cd-4TP-1 | 728.6 | 1 | 0 | 2.7 | – | Post-combustion | Pachfule and Banerjee (2011) |
| MIL-101(Cr/EDTA-Ac) | 1259 | 1 | 25 | 2.46 | CO2/N2: 9.46 | Post-combustion | Chen et al. (2018a) |
| Ni-MOF-74 | – | 0.7 | 25 | 5.2 | – | – | Bae et al. (2014) |
| MIL-101(Cr) | 495.23 | 2 | 25 | 5.7 | CO2/N2: 57 | Post-combustion | Lin et al. (2014) |
| MIL-101(Cr,Mg) | 3274 | 1 | 25 | 3.28 | CO2/N2: 41 | Post-combustion | Hu et al. (2014) |
| BUT-161 | 308 | 1 | 25 | 2.14 | O2/N2: 57 & CO2/CH4: 10 | Post-combustion and biogas upgrading | Zhang et al. (2019) |
| MAF-X27(ox) | 1167 | 1 | 25 | 6.7 | CO2/N2: 262 | Post-combustion | Liao et al. (2015) |
| Mg-MOF-74 | 1174 | 1 | 25 | 8.6 | CO2/CH4: 10.1 | Post-combustion and biogas upgrading | Bao et al. (2011) |
| Co-MOF-74 | 957 | 1 | 25 | 7.5 | – | – | Yazaydin et al. (2009) |
| Al(HCOO)3(ALF) | – | 1.18 | 50 | 4.3 | CO2/N2: 350 | Post-combustion | Evans et al. (2022) |
| MOF-177-EDTA-20% | 855 | 1 | 25 | 2.83 | – | – | Gaikwad et al. (2021) |
| MOF-177-TEPA-20% | 585 | 1 | 25 | 3.82 | – | – | Gaikwad et al. (2021) |
| MW-180–30 | – | 1 | 25 | 2.02 | CO2/N2: 29.7 | Post-combustion | Chen et al. (2019) |
| MOF-1 [Ni-(4PyC)2·DMF] | – | 1 | 30 | 4.1 | CO2/N2: 82 | Post-combustion | Nandi et al. (2017) |
MOFs, metal–organic frameworks; BET, Brunauer; Emmett and Teller technique. CO2, carbon dioxide; N2, nitrogen; and CH4, methane
aAll reported adsorbents are commercial and/or patented names
Beside the promising characters of metal–organic frameworks, which are highly interesting for carbon dioxide capture studies, one of the major concerns related to the large-scale applications of these adsorbents is the cost (Younas et al. 2020). Accordingly, in the synthesis step, the solvent cost dominates the total cost of production (Younas et al. 2020). Quantitatively, aqueous synthesis of metal–organic frameworks associates with lower net price of 13–36 $/kg of adsorbent, which is much lower than the conventional method of solvothermal with an average price of 35–75 $/kg (Younas et al. 2020). Nevertheless, the other steps of the carbon dioxide adsorption process via metal–organic frameworks including adsorption and regeneration are comparable to the conventional adsorbents (Younas et al. 2020).
Further, the instability under humid operating conditions is the other drawback of these sorbents, which has diminished the application range of this adsorbent, practically. On the grounds, numerous researchers have attempted to develop the metal–organic frameworks with characteristics as water-resistant adsorbents (Ding et al. 2016; Vakili et al. 2018; Palakkal and Pillai 2022). In this way, high oxidation state metals utilization and metal sites and/or organic linkers replacement with hydrophobic substances are among the possible solutions (Vakili et al. 2018). In recently attempts, composite forms of metal–organic frameworks with novel substances have presented appealing adsorption properties for carbon dioxide capture (Ding et al. 2016; Palakkal and Pillai 2022), which refer to a new window toward the metal–organic frameworks characteristics optimization.
Additionally, polyethylenimine (Tao et al. 2013), polynaphtylene (Ding et al. 2016), 1-ethyl-3-methylimidazolium thiocyanate (Manuel Vicent-Luna et al. 2018), benzoic acid-functionalized graphene (Kumar et al. 2016), mesoporous silica (Chakraborty and Maji 2014), graphene oxide (Liu et al. 2013a), and carbon nanotube (Xiang et al. 2011) have been utilized as modifier in metal–organic frameworks structure. Permyakova et al. (Permyakova et al. 2017) lately developed Al dicarboxylate metal–organic frameworks denoted MIL-160(Al) as a water-stable sample using high valence metal cations, which has already demonstrated interesting results for gas separation and purification studies.
Further, incorporating this type of adsorbent with mixed matrix membranes is the other technique, which has been considered to develop high-efficient adsorbents with supreme selectivity and productivity characters (Pettinari and Tombesi 2020). A summary of pros and cons of metal–organic frameworks for gas separation and purifications applications is described in Table 10.
Table 10.
Advantages and disadvantages of metal–organic frameworks (Wang et al. 2010; Lee and Park 2015; Younas et al. 2020; Palakkal and Pillai 2022)
| Advantages | Tunable surface characteristics |
|---|---|
| Incorporation with other modifiers | |
| As additive to modify the capacity of other technologies | |
| Catalytic characteristics for CO2 conversion | |
| Reasonable regenerability | |
| High porosity | |
| Disadvantages | Instability in humid condition |
| Cost issues | |
| Storage capacity | |
| Encapsulation of molecules with larger size | |
| Lower mechanical stability in higher porosity | |
| Negligible capacity in low pressure | |
| Complicated synthesis process |
Covalent organic frameworks
Basically, covalent organic frameworks are crystalline highly porous polymers and organic materials linked by strong covalent bonds via π-structure forms (Côté et al. 2005; Feng et al. 2012). Covalent organic frameworks as versatile materials with tunable pore size and specific thermal and chemical stability represented significant characteristics in carbon dioxide adsorption purposes (Gole et al. 2018). In general, they follow a repetitive pattern of organic materials and exhibit high surface area, satisfying density, numerous functional groups adaption whereas can be occasionally tuned (Gole et al. 2018).
One of the first synthesis of covalent organic frameworks was attributed to Côté et al. (2005) in 2005. The two-dimensional COF-1 and COF-5 were synthesized via solvothermal method using phenyl diboronic acid and hexahydroxytriphenylene as the building blocks (Côté et al. 2005). After the prosperous study of Cote et al. (2005), other researchers also developed the covalent organic frameworks with novel ingredients to develop more efficient adsorbents for gas separation studies (Wang et al. 2021a; Lyu et al. 2022; Sani et al. 2022). Accordingly, some studies evaluated the covalent organic frameworks enriched by nitrogen sites (Lyu et al. 2022). These types of sorbents demonstrated significant affinity toward carbon dioxide molecules.
Lyu et al. (2022) also developed a novel covalent organic framework incorporated with reactive aliphatic amine materials including 3-aminopropyl amine, which led to synthesize a more efficient adsorbent with 29% higher capacity than pristine framework and more stable toward water presence. High affinity toward carbon dioxide contributed to the introduction of this novel adsorbents as an attractive adsorbent candidate in low carbon dioxide concentration conditions, specifically direct air capture (Lyu et al. 2022). However, cost issue is still one of the main concerns for large-scale applications of this class of sorbents (Sani et al. 2022).
Typically, covalent organic frameworks are synthesized through various methods, which the main ones accompanied with advantages and disadvantages illustrated in Table 11 (Feng et al. 2012; Wei et al. 2015). It is noteworthy that solvothermal is the most popular and the most reliable one among the reported covalent organic frameworks synthesis methods (Wei et al. 2015). Table 12 also demonstrates a comparison between some of recent developed covalent organic frameworks for carbon dioxide adsorption.
Table 11.
Advantages and disadvantages of synthesis methods for covalent organic frameworks (Feng et al. 2012; Wei et al. 2015)
| Method | Advantages | Disadvantages |
|---|---|---|
| Solvothermal | Maturity | Synthesis problems with fully soluble and insoluble building blocks |
| Microwave | Rapid reaction | Used for COF-5a and COF-102a only |
| Simple large-scale production | ||
| No requirement to sealed vessel | ||
| Better porosity | ||
| Ionothermal | High temperature requirement | – |
| Building block selection limitation | ||
| Synthesis on metal surfaces | Large-scale production | Requirement to precise reaction condition |
| High purity of building blocks | ||
| Utilization of a single-crystal metal surface | ||
| Synthesis on HOPG surfaces | The presence of CuSO4.5H2O as a water reservoir | – |
| Synthesis on graphene surfaces | – | Unmatured |
HOPG, Highly ordered pyrolytic graphite; CuSO4.5H2O, copper sulfate pentahydrate; COF, covalent organic frameworks
aRepresents the commercial name of covalent organic frameworks
Table 12.
Comparison of recently developed covalent organic frameworks for carbon dioxide adsorption
| COFsa | Surface area (m2/g) | Temperature (◦C) | Pressure (bar) | CO2 uptake (mmol/g−1) | Selectivity | Potential application | References |
|---|---|---|---|---|---|---|---|
| COF-10 | 1760 | 0 | 1 | 1.2 | CO2/CH4: 2 | Biogas upgrading | Furukawa and Yaghi (2009) |
| COF-10 | 1760 | 25 | 5 | 3.41 | CO2/CH4: 7.5 | Biogas upgrading | Furukawa and Yaghi (2009) |
| SQ-COP-1 | 8700 | 25 | 3 | 3.29 | CO2/CH4: 4.14 & CO2/N2: 4.83 | Biogas upgrading and post-combustion | Huang and Cao (2016) |
| SQ-COP-3 | 8500 | 25 | 3 | 1.93 | CO2/CH4: 8.5 & CO2/N2: 7.08 | Biogas upgrading and post-combustion | Huang and Cao (2016) |
| TDCOF-5 | 2050 | 0 | 1 | 2.09 | CO2/CH4: 2.91 | Biogas upgrading | Kahveci et al. (2013) |
| ILCOF-1 | 2723 | 0 | 1 | 1.38 | CO2/CH4: 2 | Biogas upgrading | Rabbani et al. (2013) |
| ILCOF-1 | 2723 | 25 | 5 | 3.8 | CO2/CH4: 2 | Biogas upgrading | Rabbani et al. (2013) |
| PCTF-4 | 1404 | 0 | 1 | 4.66 | CO2/CH4: 20 & CO2/N2: 56 | Biogas upgrading and post-combustion | Gu et al. (2015) |
| FCTF-1–600 | 1535 | 0 | 1 | 5.53 | CO2/N2: 19 | Post-combustion | Zhao et al. (2013) |
| CTF-TPC | 1668 | 0 | 1 | 4.25 | – | – | Zhang et al. (2011) |
COF, covalent organic frameworks; CO2, carbon dioxide; N2, nitrogen; and CH4, methane
aAll reported adsorbents are commercial and/or patented names
However, while this type of sorbent received a specific interest for gas adsorption applications, but still the synthesis methods suffer from maturity and reliability that requires more efforts for developing novel synthesis methods and discovering new linkage reactions to expand the ability of these materials for carbon capture in more efficient way (Feng et al. 2012; Wei et al. 2015). Finally, Table 13 compares the key characters of activated carbons, zeolites, metal–organic frameworks and covalent organic frameworks for carbon dioxide sequestration purposes.
Table 13.
Carbon dioxide adsorbent characteristics (Choi et al. 2009; Wang et al. 2010; Olajire 2017; Younas et al. 2020; Usman et al. 2021; Karimi et al. 2022b)
| Adsorbent | Regenerability | CO2 capture capability | H2O negative effect | Selectivity | Cost |
|---|---|---|---|---|---|
| Zeolite | Intermediate | High | High | Intermediate | Low |
| Activated carbons | High | Intermediate | Low | Low | Intermediate |
| Metal–organic frameworks | High | High | High | High | High |
| Covalent organic frameworks | Intermediate | High | High | High | High |
CO2, carbon dioxide and H2O, water
Methods to validate adsorbent materials
As already discussed, there has been phenomenal growth in the development of adsorption techniques and adsorptive technologies regarding the increasing demand for energy and the environmental challenges (Sircar 2006). Accordingly, researches and studies on adsorption technology span a variety of end-use applications such as carbon dioxide capture from post-combustion processes (Liu et al. 2021a), gas sweetening (Tagliabue et al. 2012), biogas upgrading (Aghel et al. 2022), air separation (Pan et al. 2017) and hydrogen storage (Yang et al. 2012), while the process conditions of each application differ (Shade et al. 2022).
Routinely, gas adsorption studies include exposing the sorbates on an adsorbent system, thereafter determining the adsorption facts including loading capacity, heat of adsorption, selectivity, kinetic, thermodynamic and finally developing cyclic adsorption processes (Karimi et al. 2022b). In this way, different methods to validate adsorbent materials can be classified in four major classes including:
-
I.
Breakthrough
-
II.
Gravimetric
-
III.
Volumetric
-
IV.
Volumetric-Gravimetric
Breakthrough technique
The history of modern breakthrough technique is back to 1952 (Bartle and Myers 2002), where Martin and James presented the separation of volatile fatty acids using chromatography considering the nitrogen gas as the mobile phase and a stationary phase of silicone oil/stearic acid (James and Martin 1952; Bartle and Myers 2002). Over the past decades, this technique has remarkably flourished to become one of the commonly used adsorption techniques.
Routinely, the breakthrough measurement begins by introducing a gas flow at a known composition to an adsorbent bed, which has already been activated using a specific procedure. In this way, different gas compositions are controlled using various mass flow controllers also another one is employed for measuring the inert gas. The experiments are run until each sorbate “breaks through” the fixed bed, which the adsorbed amount is determined by integrating the difference between the inlet and outlet molar flow rates of each sorbate over the time.
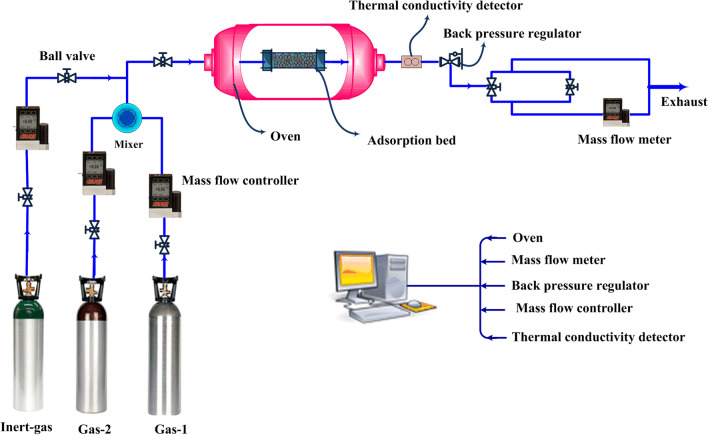
Accordingly, highly accurate detectors are required to monitor the composition of the effluent gas that gas chromatograph and mass spectrometer are among the most popular ones (Shade et al. 2022), while other sensors like infrared (Vivo-Vilches et al. 2018) or hydrogen analyzers (Danaei Kenarsari et al. 2013) have practical usages. It is noteworthy, the total pressure during the adsorption process is controlled using a back-pressure regulator (Bastos-Neto et al. 2011). A simple schematic of this technique is demonstrated in Fig. 9.
Fig. 9.
Breakthrough measurement apparatus for gas adsorption studies. This technique is the most favorable method for measuring the gas adsorption mixtures, while it also requires the highly accurate detectors
Generally, the breakthrough technique is the most popular one for gas separation and purification studies, whereas it has its own pros and cons. On the grounds, the flexibility of this technique is very high, and relatively simple as well as it has the benefit of ease of use even in the scale of less than gram of sorbent, which nominate the breakthrough method as the most favorable one for measuring the gas adsorption mixtures (Keller and Staudt 2005; Pullumbi et al. 2019; Shade et al. 2022).
However, it has some drawbacks and limitations. Firstly, the breakthrough techniques are commonly provided commercially, which these apparatuses require relatively a high budget (Keller and Staudt 2005). Also, the challenge of variations in the effluent stream raises some concerns about the capacity of this technique, while employing an inert carrier gas has been a helpful strategy (Keller and Staudt 2005).
In addition, the accurate breakthrough measurement strongly relies on the precise knowledge on the dead volume of fixed-bed column that can nearly be determined by adsorption tests using sand or glass beads so-called “blank-experiments”. Furthermore, regarding the multi-components gas experiments the users require to be informed about the roll-up concepts (Kapoor and Yang 1987; Li et al. 2011). But the biggest critique of breakthrough technique is related to doing one breakthrough experiment for one equilibrium data whereas for the next one the sample shall be regenerated, causing this method relatively time consuming and inappropriate for collecting large data sets (Shade et al. 2022).
Gravimetric technique
Gravimetric technique is the most direct adsorption method for the assessment of loading capacity of sorbates, which relies on a little required calculation (Lachawiec et al. 2008). The principal concept behind this technique is dating back to the ancient eras, when the mass assessment through weighing using Earth’s gravity was firstly performed, while employing this phenomenon in the gas adsorption onto the solid sorbents is back to the second half of nineteenth century (Keller and Staudt 2005).
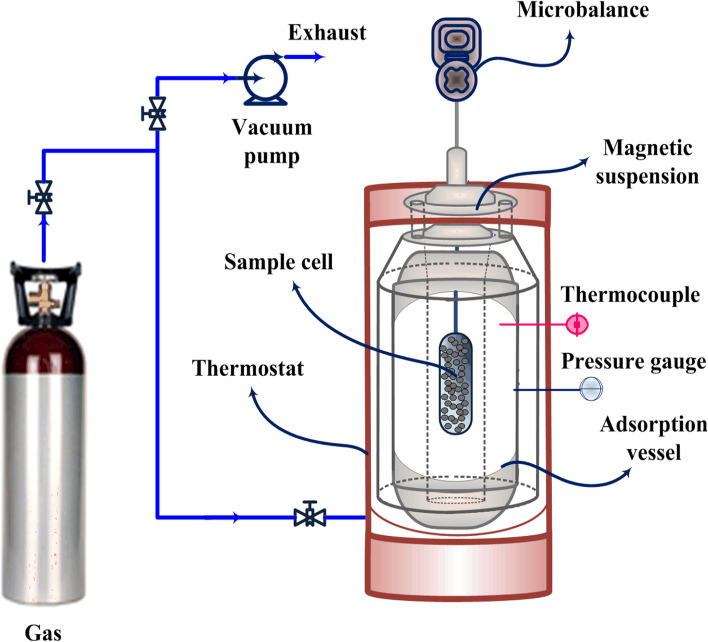
Generally, to accomplish a gravimetric experiment, a cell that is equipped with a sensitive microbalance is loaded with a small amount of adsorbent, afterward, the adsorbing gas is introduced to the system (Minnick et al. 2018). Accordingly, the total mass adsorbed is determined by calculating the sample weight variations. The principle of the gravimetric measurements is illustrated in Fig. 10. As can be found, this technique is proper for pure gas adsorption, whereas for gas mixture the system shall be coupled with one of other methods. It is worth noting that different variants can be specified regarding the considered balance as a single or double beam type (Keller and Staudt 2005; Shade et al. 2022).
Fig. 10.
Gravimetric unit for gas adsorption studies using a magnetic suspension balance. This technique is the most direct adsorption method for the assessment of loading capacity of sorbates, which relies on a little required calculation
Recently some developments on gravimetric methods have been introduced such as gravimetric—densimetric method and the gravimetric—Van Ness (Shade et al. 2022). Also, the employed integral mass balance method is one of the most exciting advancements on this technique (Broom et al. 2020). Although it can also be considered as an addition of gravimetry to the breakthrough one for measuring the “stepwise” breakthrough profiles without the requirement of sample regeneration for getting the adsorption equilibrium data sets of each isotherm (Broom et al. 2020).
However, the gravimetric adsorption measurement owns several benefits as well as some significant drawbacks, which requires to be distinguished before conducting the experiments (Paswan et al. 2004; Yang et al. 2016; Pan et al. 2017). Accordingly, the buoyant effects are essential to be corrected for gas adsorption, while it is relatively easy for pure gas assessment, but some complexities emerge in the case of multi-components adsorption concerning the density of gas (de Weireld et al. 2005; Nguyen et al. 2017). As getting the gravimetric equilibrium data may last hours or even days, accordingly a gas circulator has been proposed in some cases to reduce the adsorption time, which may cause some difficulties for fine grained adsorbents (Keller and Staudt 2005).
In addition, the cost and sophistication of such a system are other drawbacks of this technique (Keller et al. 2002; Paswan et al. 2004). Furthermore, the temperature control is more complicated concerning the sorbent being on a balance (Paswan et al. 2004; Fakher and Imqam 2020). Besides the mentioned barriers and limitations of gravimetric technique, it has already proved some singular benefits. Generally, this method is the most popular one after the breakthrough technique for gas adsorption studies. Also, as already mentioned, this method is the most direct one for the acquisition of adsorption equilibrium data, while it represents the most reliable values regarding the high-pressure experiments (Shade et al. 2022).
On the other hand, the gravimetric measurement gives the opportunity of sample evaluation in the milligram scale, which is beneficial in the cases of novel adsorbents such as metal–organic frameworks and covalent organic frameworks concerning their cost, synthesis time and loading capacity (Karimi et al. 2021d). Novel gravimetric apparatuses represent very high accuracy accompanied with the availability of kinetic determination, thanks to remarkable advances on weighing techniques and modern precise microbalances (Fakher and Imqam 2020).
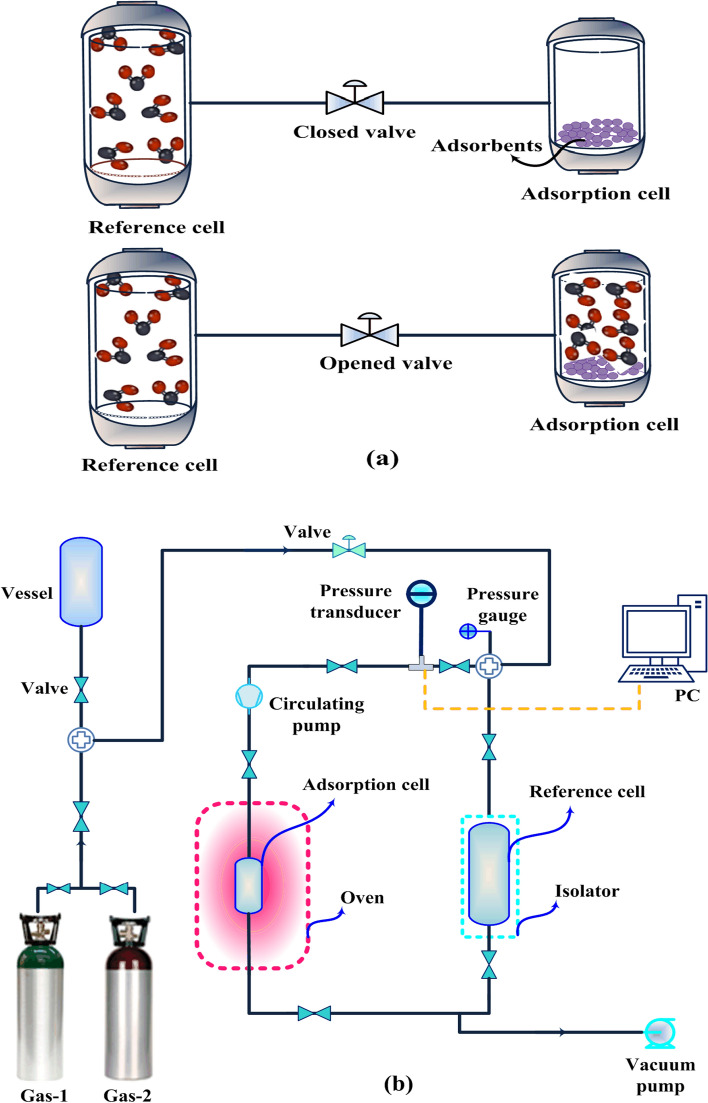
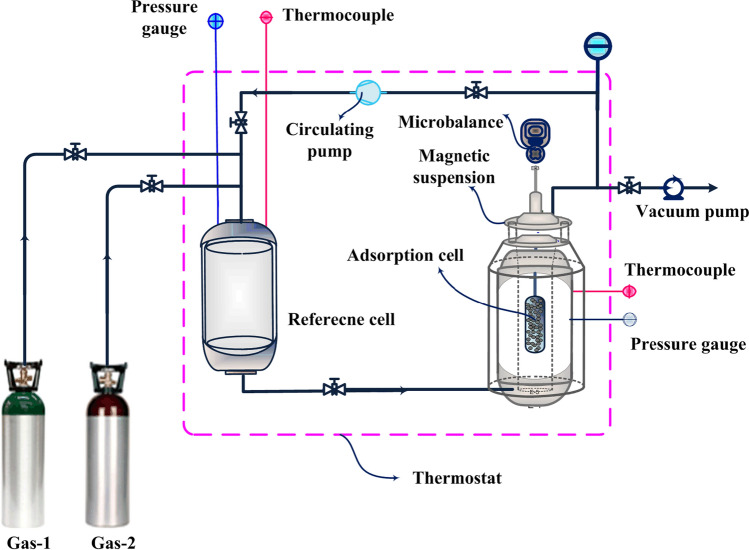
Volumetric technique
The gas adsorption measurement using volumetric technique was firstly developed by Sieverts (1907), where designed a glass volumetric unit for assessment of gas absorption and diffusion. During the last decades, this primary apparatus has passed its evolution to become one of the extremely popular techniques for different gas adsorption processes (Dreisbach et al. 1999; Lachawiec et al. 2008). Typically, a volumetric system consists of an adsorption cell, a reference cell, thermometers, pressure transducer and a source of energy provider, based on considered application (Paswan et al. 2004).
The experimental procedure starts by pressurizing the reference cell with gas sorbate, thereafter, expansion to the adsorption cell, which has already been loaded with a known mass of activated adsorbent. During the experiment, the pressure variations are recorded until getting the equilibrium conditions that allows the determination of adsorbed amount using an equation of state (Keller et al. 2002; Paswan et al. 2004; Fakher and Imqam 2020). Accordingly, based on to the nature of this technique, there is some arguments that the name of “manometric” is more suitable than “volumetric” for such a system, because the key variable is the pressure and not the volume (Keller and Staudt 2005).
It is noteworthy, performing the experiments may last milliseconds, minutes, hours or even days, whereas it depends on type of adsorbent and the dynamic of instrument (Keller and Staudt 2005; Karimi et al. 2021d). On the other hand, acquiring precise equilibrium data strongly relies on well calibrating/knowing different volumes of apparatus (Shade et al. 2022). A principle of volumetric measurement is illustrated in Fig. 11a. Also, a simple schematic of this method is depicted in Fig. 11b. Commonly, this technique is more popular for pure gas adsorption assessment; however, some convolutions emerge for multi-component gas adsorption, which requires employing other analytical systems such as the gas chromatography for analyzing the gas compositions. In this way, some interesting practical applications of volumetric measurements of gas mixture have been studied in slurries (Zhang et al. 2015) and fluidized beds processes (Costa et al. 1981).
Fig. 11.
a A principle of volumetric measurement for the assessment of gas adsorption, b a simple volumetric apparatus for gas adsorption measurements. Only practical for single component gas adsorption studies. PC, personal computer
One of the main advantages of volumetric units is its simplicity, which can be provided commercially or in-house built. Typically, the commercial instruments have some restrictions including the size and the ratio of adsorption cell to the reference cell, type of studied sorbents such as pellets, beads, powder, and carbons monoliths, as well as the ranges of pressure and temperature, for example through cryogenic studies, using large amount of sample than breakthrough and gravimetric, usually several grams. Also in some cases, requires a high budget for supplying (Mason et al. 2015).
Accordingly, recently, Karimi et al. (2021d) designed and developed a fast kinetic volumetric apparatus for gas adsorption in the milligram of adsorbent that demonstrated the potential of data acquisition from vacuum till high pressure. In addition, it has the flexibility of isotherm assessment in routine temperatures of post- and pre-combustion processes or to be upgraded for cryogenic studies, accompanied with potential of different ratios of adsorption and reference cells. In general, in-house built volumetric units are among the less expensive adsorption apparatuses, where the experiments can be simply run without any sophistication or requirement for permanent supervision (Keller and Staudt 2005).
Volumetric-gravimetric technique
To remove the barriers of multi-component gas adsorption the volumetric-gravimetric measurement was developed to have the benefits of both these techniques and allow the feasibility of binary co-adsorption without the necessity of employing routine gas analyzers such as a mass spectrometer or gas chromatograph. Accordingly, it can be classified in either the volumetric group or the gravimetric one (Keller and Staudt 2005).
Firstly, Bering and Serpinskij introduced this method to specify the adsorption equilibria values of gas–solid mixture systems without external intervention for sampling (Bering and Serpinskij 1953), while the technique was not widely used at the beginning because of the requirement of sufficiently different molecular weights (Bülow 2022). Later, some improvements were employed on this technique by Bülow et al. (Bülow 2022) regarding the adsorption study of binary hydrocarbon mixtures at the high temperatures. Finally, the technique was commercialized by Keller et al. (1992).
Since then, some other modifications have been introduced to this method for adsorption assessment of more than two components in the gas mixture (Hamon et al. 2014; AbdulKareem et al. 2018). Currently, it has been admissible one in different areas including hydrogen storage (Suyetin 2017), greenhouse gases separation (Yang et al. 2015), and adsorption of light olefin/paraffin mixtures (Hovestadt et al. 2018).
Routinely, volumetric-gravimetric measurement avoids the direct measuring of gas phase compositions. On the grounds, the normal volumetric method is considered to specify the total molar quantity adsorbed onto the sample at the studied temperature and pressure, while the mass of the adsorbed phase is obtained using gravimetric assessment after considering the required corrections on the buoyant force (Keller et al. 2002). Eventually, the partial loading of each sorbate can be simply calculated by knowing the molar value adsorbed and the adsorbent mass (Keller and Staudt 2005; Shade et al. 2022).
An illustration of the volumetric-gravimetric adsorption measurement technique is demonstrated in Fig. 12. Accordingly, since both pressure variations and weight changes are continuously recorded, this technique is extremely accurate for gas adsorption studies (Fakher and Imqam 2020). On the other hand, employing pressure transducer and microbalance simultaneously causes some complexity for new setups. In addition, to obtain precise volumetric measurement a large volume of adsorbent is required, which is the other drawback of this technique. However, volumetric-gravimetric technique is one of the favorable methods for industrial applications (Keller and Staudt 2005). A comparison between the pros and cons of different adsorption techniques is summarized in Table 14.
Fig. 12.
The volumetric-gravimetric technique for gas adsorption measurement. It has the benefits of both volumetric and gravimetric techniques, which allows the feasibility of binary co-adsorption without the necessity of employing routine gas analyzers such as a mass spectrometer or gas chromatograph
Table 14.
Comparison of adsorption techniques
| Techniques | Advantages | Disadvantages |
|---|---|---|
| Breakthrough | Highly flexible | Inappropriate for collecting large data sets |
| User friendly | Relatively expensive | |
| Easy to use even in the scale of less than gram | Requires highly accurate detectors | |
| Employed technique for developing cyclic adsorption processes | Requires precise knowledge on the dead volume of fixed-bed column | |
| Gravimetric | The most direct adsorption technique | Only proper for pure gas adsorption |
| The opportunity of adsorption screening in the milligram scale | Influenced by buoyant effects | |
| The potential of highly reliable values for high-pressure experiments | Getting the gravimetric equilibrium data may last hours or even days | |
| The cost and sophistication of system | ||
| Volumetric | Simplicity | Acquiring precise equilibrium data strongly relies on well calibrating/knowing different volumes of apparatus |
| It can be provided commercially or in-house built | Generally, getting the equilibrium values may last hours or even days | |
| In-house built units can be developed with low budgets | More popular for pure gas adsorption assessment | |
| The most common technique for collecting large data sets of equilibrium values | ||
| Volumetric-Gravimetric | Extremely high accuracy | Some complexity for doing the experiments |
| Proper for multi-component gas adsorption | Commonly, a large volume of adsorbent is required | |
| Favorable method for industrial applications | Relatively an expensive technique |
Cyclic adsorption processes
After obtaining the adsorption equilibrium information using different adsorption techniques, it is required to design a cyclic adsorption process for evaluating the separation performance of sorbents concerning the large-scale applications. Accordingly, regarding the determinative variables of adsorption equilibrium process, one can develop a “swing” adsorption process using temperature and pressure (Karimi et al. 2022b). To this end, the saturated fixed-bed column is regenerated by varying one of the process variables, and these cycles pursue through the specific sequences until getting a cyclic steady-state process (Karimi et al. 2022b).
The concept of cyclic adsorption process was firstly introduced by Charles Skarstrom in 1932, by developing pressure swing adsorption, while it was patented thirty years later in 1960 (Skarstrom 1960), after patenting the temperature swing adsorption process (Kahle 1950), because of that, it was initially known as “heatless” process (Grande 2012). The “Skarstrom cycle” contains four main steps including feed, blowdown or evacuation, purge and pressurization, which its simple schematic is illustrated in Fig. 13. The fundamental character of PSA process specifies by getting the required driving force for gas separation by swinging the pressure at the high and low domains. In this way, after sorbent saturation in the column, the pressure in the column is reduced, which results in a partial desorption of sorbates loaded in the bed (Grande 2012).
Fig. 13.
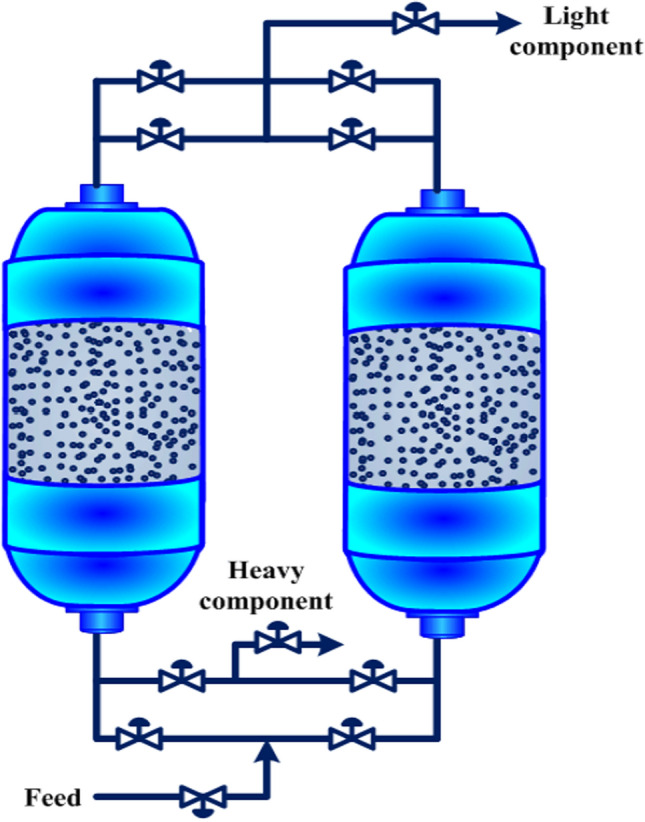
The first two-column pressure swing adsorption unit of Charles Skarstrom, which includes four main steps such as feed, blowdown or evacuation, purge and pressurization
Through the years, this technology has been employed in different areas including methane upgrading (Ferreira et al. 2015), carbon dioxide removal (Karimi et al. 2020b), air separation (Arvind et al. 2002), noble gases purification (Jahromi et al. 2018), hydrogen purification (Luberti and Ahn 2022), and normal-iso paraffin separation (Dobladez et al. 2020). Hence, currently, the major industrial application of pressure swing adsorption process is related to the hydrogen purification and air separation, specifically for medical application of oxygen production (Grande 2012).
Temperature swing adsorption as other well-known cyclic adsorption process was almost patented at the same time of pressure swing adsorption process by Kahle (1950). In this technology, the adsorption–desorption steps are developed by swinging the temperature at the high and low values (Karimi et al. 2022b). Generally, developing the temperature swing adsorption cycles last for several hours, while the pressure swing adsorption cycles are very shorter.
Further, electric swing adsorption is a special case of temperature swing adsorption process in which, the desorption is accomplished by raising the temperature of sorbents with passing the electricity through a conductor using the Joule effect (Petkovska et al. 2006; Grande et al. 2009). In fact, the regeneration step is achieved by decreasing the equilibrium capacity of samples (Grande et al. 2009; Ribeiro et al. 2014), which this process has some benefits comparing conventional temperature swing adsorption such as: higher heating efficiency because of direct delivery of energy to the sorbents, faster heating rate, mass and heat fluxes being in the same direction, which are advantages for the performance of desorption step (Grande et al. 2009; Ribeiro et al. 2014).
The years after inventing Skarstrom cycle, several other patents and modifications were introduced to improve the performance of this process. In this way, vacuum pressure swing adsorption was presented by Guerin de Montgareuil and Domine, to perform the regeneration under the vacuum (de Montgareuil and Daniel 1958). To this end, the desorption step is commonly accomplished at 0.01–0.1 bar, while the sorbate is inset to the column at the atmospheric pressure or slightly higher than 1 bar (Ho et al. 2008; Liu et al. 2011). However, vacuum employing probably increases the energy consumption of system, but if the uptake capacity varies significantly at the lower pressure of 1 bar, the efficiency of process may dramatically be improved (Grande 2012).
In other modifications on Skarstrom cycle, some considered the pressurization step using part of the enriched gas to boost the purity of the produced gas (Ahn et al. 1999). Further, ESSO research group proposed a pressure equalization step to employ a part of gas, which is normally lost in the blowdown step, to pressurize the other column for enhancing purified gas production (Stark 1963). This improvement in the conventional 2-column pressure swing adsorption unit contributes to a remarkable impact on the “continuity” of the process. Accordingly, during the pressure equalization of two columns, at least one more column is necessary for feed processing (Stark 1963), while employing several columns provide the possibility of different pressure equalization steps, consequently, the total recovery more raised (Xu and Weist 2000; Jianguo et al. 2001).
Basmadjian and Pogorski (1963) defined a new step as “rinse” considering a recycle of the heavy component by displacing the light component to the product end. Nevertheless, this advancement was firstly introduced as an approach to the concentration of low percent light compounds, but it has also been accepted for other purposes (Fuderer 1983; Na et al. 2002). In this way, Knaebel (Kent 2009) patented moving bed type of temperature swing adsorption for improving the efficiency of traditional type of temperature swing adsorption.
Additionally, in one of the most recent inventions on cyclic adsorption processes, Svante’s carbon capture technology (SvanteInc 2021) structured adsorbent laminate, a rotary mechanical contactor and a proprietary process cycle design to capture carbon dioxide from the flue gas and regenerate the adsorbent in a single unit, all in 60 s.
Beside the advances of different types and configurations in cyclic adsorption processes, other progress can be classified in two major classes including: material science concerning the discovery of highly efficient sorbents for employing in such processes, and engineering science regarding the determining optimal approaches to improve the performance of process, which these concepts are extensively addressed in the next sections. However, the question that was planned in this area by Professor Ruthven in 1992 has not completely been answered by adsorption community till now, which raised: “Is it possible to develop an algorithm for automatic generation of pressure swing adsorption cycles and tuning of the various steps?” (Ruthven 1993).
It is worth noting that as a general principle, pressure swing adsorption process has some preference than other cyclic adsorption processes regarding its simplicity, flexibility, and low energy consumption, especially when the concentration of removed component is quite significant, which contributes to a balance between the time of adsorption and desorption steps (Ho et al. 2008; Grande 2012). On the other hand, when the removed component has a low concentration, other options such as temperature swing adsorption and electric swing adsorption can be evaluated, because the adsorption step may last for a while (Humphrey and Keller 1997).
Direct air capture
Despite all attempts to reduce carbon dioxide, regrettably gigatons of carbon are annually released to the atmosphere (Fuss et al. 2018; Minx et al. 2018). Routinely, the conventional methodologies such as post-combustion, pre-combustion, and oxy-fuel combustion processes (Karimi et al. 2021c) are trapping carbon dioxide from the stationary sources in the petroleum refineries and petrochemical complexes, coal power plants as well as cement, iron, and steel industries (Karimi et al. 2014, 2018a).
However, technologies related to the noncarbon energy have quickly grown in the recent years, but unfortunately, the corresponding progress does not show a promising perspective considering the global energy demand (Terlouw et al. 2021b). On the grounds, the recent summit of climate changes mitigation in Paris declared a rapid and urgent development of “negative carbon technologies” are required (UNFCCC 2022), a subject which was re-stated by the National Research Council on Climate Intervention (Council 2015). Generally, carbon dioxide removal technologies, or negative emission technologies are ascribed to the technologies, which are able to remove and eliminate available carbon dioxide from the atmosphere (Terlouw et al. 2021b).
According to the defined projections, carbon dioxide removal technologies are planned to remove 20 gigatons of carbon annually by 2050 (Fuss et al. 2018; Minx et al. 2018). Nevertheless, there are some uncertainties and doubts related to this value (Fuss et al. 2018; Minx et al. 2018). However, proposed different energy scenarios developed by integrated assessment models specified that without extensive development of carbon dioxide removal technologies, it is infeasible achieving the Paris agreement target for 1.5 °C reduction in global temperature (Smith et al. 2015).
In this way, recently several different technologies concerning carbon dioxide removal have been evaluated including ocean fertilization, biochar, enhanced weathering, bioenergy with carbon capture and storage, and direct air carbon capture (Fuss et al. 2018; Minx et al. 2018; Terlouw et al. 2021b). Nevertheless, regarding some skepticisms about environmental side effects of carbon dioxide removal technologies (Terlouw et al. 2021b), direct air capture process represents an appealing potential for directly capture carbon dioxide from atmosphere, which has already been emitted (Smith et al. 2015; Fuss et al. 2018; Minx et al. 2018; Terlouw et al. 2021b). On the grounds, direct air capture technology accompanied with renewable sources of energy can have remarkable roles toward a decarbonized world.
The concept of direct air capture for employing in climate change mitigation was firstly proposed by Lackner in 1999 (Lackner et al. 1999). Later in 2007, Global Thermostat patented the direct carbon dioxide capture from air with amine-modified samples (Eisenberger and Chichilnisky 2007). Next, in 2012, Global Thermostat introduced an air contactor, which is able to operate with many different sorbents (Eisenberger 2012). During the last decade, numerous other investigations and analyses have been accomplished to evaluate the feasibility and viability of this strategy for carbon capture.
Routinely, direct air capture, alike other conventional carbon capture processes, contains two main steps including adsorption or absorption step and desorption step, which in this case, because of the highly dilute concentration of carbon dioxide in the atmosphere, is a challenging subject. It is noteworthy, today, carbon dioxide concentration is around 419 ppm in the atmosphere (NASAClimate 2022b), which is around 350 times lower than available one in the flue gas of coal power plants (Christopher 2011). Hence, the traditional sorbents for gas adsorption applications, such as zeolites, and activated carbons, which represent physisorbent and chemisorbent characterizations, demonstrate unfavorable performance at the low partial pressures with a small loading capacity and very low selectivity for carbon dioxide capture (Takeda et al. 2012).
In this way, the aqueous amine solutions as oldest technique for carbon dioxide capture and sequestration from gas streams came back to the game for such a purpose (Didas et al. 2015). In addition, the potential of solid-supported amine sorbents for direct air capture have been extensively investigated in many studies (Caplow 1968; Danckwerts 1979; Takeda et al. 2012; Didas et al. 2015). These adsorbents because of strong bonds among the carbon and the amines as a result of a chemical reaction, demonstrate an acceptable loading capacity, heat of adsorption and selectivity even at the low concentrations of carbon dioxide (Caplow 1968; Danckwerts 1979).
Thus, the supported organic–inorganic hybrid samples with amines can be a proper candidate for direct air capture. Accordingly, recently, metal–organic frameworks as a novel class of adsorbents received a significant attention, because of versatility, highly ordered, functionality, and tenability characters (Shekhah et al. 2014). It is noteworthy that among the numerous synthesized metal–organic frameworks till now, MOF-74 is one of the most studied sorbents for such a purpose (Wang et al. 2014a; Shekhah et al. 2014).
As any other emerging technology, the direct air capture possesses its own decisive benefits and probably some drawbacks and uncertainties that requires more investigations. To briefly discuss, this technology has the advantage of capturing carbon dioxide from distributed sources, which are responsible for around 50% of annual carbon emissions (Schmidt et al. 2019). Further, it would not be limited to a specific location because of flexibility on installing the equipment. Additionally, the performance of process is not influenced with other contaminants that are some of available difficulties in other processes (Terlouw et al. 2021b).
Besides the many advantages, there are still some major concerns regarding the DAC, which require to be addressed for further proceeding of this technology. As stated earlier, having a promising sorbent with a rational cost concerning the uptake capacity, heat of adsorption and selectivity of carbon dioxide at very low partial pressure has remained as an obstacle. Additionally, highly energy consumption during the regeneration steps, up to 900 °C for aqueous solutions and about 100 °C for solid sorbents, is one of the other main drawbacks of this technology (Fasihi et al. 2019).
The direct air capture is usually compared with post-combustion process, while it seems an analogy deprived from relevance, because these technologies are two different processes with two different scopes, which one of them traps carbon dioxide from emission to the atmosphere, while the next one remove carbon dioxide from the atmosphere. This issue has been the place of argument in the literature during the recent years (Zeman 2007; House et al. 2011; Lackner 2013; Ruthven 2014).
In this way, the thermodynamic efficiency has been considered as a factor to evaluate the feasibility of direct air capture. On the grounds, the second thermodynamic law has been employed to evaluate the actual required energy to carry out a direct air capture system than a conventional flue gas carbon dioxide capturing process. The results showed the separation of carbon dioxide from a 400-ppm stream requires around 20 kJ/mol as minimum of the theoretical work (Zeman 2007; House et al. 2011), while it is 8.4 kJ/mol for carbon dioxide capturing from a typical flue gas using aqueous monoethanolamine (Zeman 2007).
In another attempt, Lackner investigated the variations in the minimum free energy related to sorption reaction for evaluating the irreversibility of process (Lackner 2013). It was revealed that the theoretically accessible yield for direct air capture can be higher than carbon dioxide capture from flue gas. In 2014, Ruthven claimed that the thermodynamic minimum work of separation does not show the actual required work for separation in highly dilute gases (Ruthven 2014). Accordingly, he suggested the “separative work” derived from the ideal cascade theory as a more meaningful factor and compared it with thermodynamic minimum work for assessing the separation performance in such processes (Ruthven 2014).
Ruthven findings demonstrated the energy consumption of direct air capture process can be 100 times higher than carbon dioxide capture by conventional process from a coal power plant (Ruthven 2014). Nevertheless, this estimation was relied on two key assumptions including physisorbent and a multistage process, while as already discussed, chemisorbents are mainly considered for direct air capture, also the cascade approach does not accurately predict the efficiency of separation process in a single stage. A detailed comparison between direct air capture and carbon dioxide capture from flue gas is illustrated in Table 15.
Table 15.
Comparison between direct air capture and carbon dioxide capture from flue gas
| Parameters | Direct air capture | Post-combustion |
|---|---|---|
| Operating temperature | 10–30 °C | 40–75 °C |
| Operating pressure | around 1 bar | Around 1–2 bar |
| CO2 concentration | around 419 ppm | 5–15% |
| Other contaminants | Very low | SOx, NOx, and Hg |
| Energy used | Any source | Fossil fuel from power plant |
| Capital cost | High | Moderate |
| Operational cost | Very high | Moderate |
| Reliability | High | High |
| Maturity | Low | High |
| Environment Friendliness | Very environment friendly | Very environment friendly |
| Objective | Negative carbon | CO2 capture from emissions |
SOx, sulfur oxides; NOx, nitrogen oxides; Hg, mercury; CO2, carbon dioxide
Currently, there are only three companies throughout the world that supply direct air capture plants. Global Thermostat, in the USA, deploys this technology in the pilot scale using amine sorbents supported on a monolithic contactor (Fasihi et al. 2019). Carbon Engineering from Canada also supplies high-temperature direct air capture units on the market of North America (Keith et al. 2018; Fasihi et al. 2019). Further, Climeworks from Switzerland, till now has developed several low-temperature direct air capture systems in some European countries including Italy, Iceland, Germany, Netherland and Switzerland (Fasihi et al. 2019; IEA 2022). Detailed descriptions of the plants, which are in operation worldwide also the projects in development are specified in Table 16 (IEA 2022).
Table 16.
Direct air capture plants in operation worldwide, accompanied with projects in development (IEA 2022)
| Direct air capture plants in operation worldwide | ||||
|---|---|---|---|---|
| Company | Country | Sector | CO2 capture capacity (ton of CO2/year) | Start-up year |
| Climeworks | Iceland | CO2 removal | 4000 | 2021 |
| CO2 removal | 50 | 2017 | ||
| Germany | Power-to-X | 3 | 2020 | |
| Power-to-X | 3 | 2020 | ||
| Power-to-X | 50 | 2020 | ||
| Power-to-X | 50 | 2019 | ||
| Power-to-X | 3 | 2019 | ||
| Power-to-X | 3 | 2019 | ||
| Customer R&D | 1 | 2015 | ||
| Switzerland | Power-to-X | 3 | 2018 | |
| Beverage carbonation | 600 | 2018 | ||
| Greenhouse fertilization | 900 | 2017 | ||
| Power-to-X | 50 | 2016 | ||
| Italy | Power-to-X | 150 | 2018 | |
| Netherlands | Power-to-X | 3 | 2019 | |
| Carbon Engineering | Canada | Power-to-X | Up to 365 | 2015 |
| Global Thermostat | US | R&D | 1000 | 2013 |
| R&D | 500 | 2010 | ||
| Direct air capture projects in development | ||||
|---|---|---|---|---|
| Project | Country | Target operation date | Capture capacity (ton of CO2/year) | CO2 use or storage |
| DAC pilot plant | Australia | 2022 | 365 | Storage: injection |
| Haru Oni eFuels pilot plant | Chile | 2022 | – | Use: synthetic fuels |
| DAC 1 project | US | 2025 | 1 million | Storage: injection |
| Air-to-fuels plant | Canada | 2026 | – | Use: synthetic fuels |
| AtmosFUEL Project | UK | 2029 | – | Use: synthetic fuels |
| Dreamcatcher project | 2026 | Up to 1 million | Storage: injection | |
| Sizewell C nuclearpowered DAC | – | 100 | Storage: injection | |
| Kollsnes project | Norway | – | Up to 1 million | Storage: injection |
| Norsk e-fuel project | 2023 | – | Use: synthetic fuels | |
CO2, carbon dioxide; R&D, research and development
Additive manufacturing
As already discussed, solid adsorbents possess numerous benefits, which nominate them as an appealing candidate for gas separation and purification applications. One of the most attractive characters of solid adsorbents is the utilizing less energy compared to conventional amine solutions employed in absorption processes (Pietschak et al. 2018). These solid-state adsorbents normally are in shape of powders and require to be in a practical form through industrial utilizations. The most common forms of these solid adsorbents are granules, pellets, and extrudates (Pu et al. 2018; Pietschak et al. 2018).
However, pellets and granules characterize charming benefits in formation process point of view, whereas operational defections like high pressure drop and resistance on mass transfer, limited their practical industrial deployments (Lawson et al. 2021). In this way, extruded structures, like monoliths, have been developed to optimize the mass transfer and adsorption column pressure drop (Rezaei and Webley 2009). Extrudates, owing to open channels across their structures, generally represent higher adsorption capacity.
On the other hand, recently metal–organic frameworks and covalent organic frameworks developed by this method are constrained only to the laboratory scale production due to a firing step, which contributes to the material burning; hence, researchers applied secondary growth of organic extrudates, which itself suffers from long duration and consequently, limited applications for large scales (Lawson et al. 2021). As mentioned earlier, the conventional processes suffer from the poor material versatility and low geometric flexibility; hence, additive manufacturing, with the aid of computer-design method, has emerged to develop scaffold geometries with high efficiency for adsorption processes (Pereira et al. 2022).
This novel technology has been introduced as a bright technique to develop the porous solids with extraordinary characteristics for adsorption processes. As we know, fabricating an engineered shape of adsorbent with formulated structure, significantly affects the adsorption efficiency, which is precisely implemented in additive manufacturing (Lawson et al. 2021). Accordingly, by using this strategy, structured scaffolds can be produced with excellent chemical and physical characteristics.
To apply this technology, a printable paste, ink, shall be manufactured, afterward, injected to the stacked layers (Lawson et al. 2021). Generally, two methods of fused deposition modeling and direct ink writing are the most-applied techniques in the additive manufacturing technology (Pereira et al. 2022). It is observed that in some operational condition, materials produced by the fused deposition modeling show instability, which limits the applications in some specific chemical processes (Pohanka 2016). On the other hand, direct ink writing can be implemented through a wide range of temperature, against the fused deposition modeling, which requires high temperature (Pohanka 2016).
It is worth noting that the direct ink writing faces some operational problems in drying step, in which the integrity of materials weakens (Zhu et al. 2015). The other methods include stereolithography, digital light processing, two-photon polymerization, selective laser melting, electron beam melting, continuous liquid interface production, selective laser sintering, binder jet printing, ultrasonic consolidation, laser engineered net shaping, electron beam additive manufacturing, laminated object manufacturing, and ink jet printing (Pereira et al. 2022).
The American Society of Testing Materials (ASTM) have categorized the additive manufacturing technology in seven different techniques including vat polymerization, powder bed fusion, binder jetting, sheet lamination, direct energy deposition, material extrusion, and material jetting (Astm). It is notable that until now, only vat polymerization and material extrusion methods are applied in adsorbent production processes (Pereira et al. 2022). A summary of the main advantages and disadvantages of additive manufacturing techniques is listed in Table 17.
Table 17.
Advantages and disadvantages of additive manufacturing technology in adsorbent development (Pereira et al. 2022)
| Advantages | Disadvantages |
|---|---|
| Low-cost raw materials | Poor surface quality in FDM techniques |
| Low-cost printers | Low accuracy in FDM techniques |
| Fast method | Post-process treatment requirement in FDM techniques |
| Large dimension material production | High temperature requirement in FDM techniques |
| Minimum material waste | Operational problem in DIW techniques |
FDM, fused deposition modeling; and DIW, direct ink writing
Regarding the novelty of this emerging technology, especially in the adsorption area, only a few studies have been devoted to carbon capture and sequestration. Table 18 specifies some studies concerning carbon dioxide adsorption by additive manufacturing adsorbents. In this way, recently, Liu et al. (2021b) studied on a procedure to develop binder-free sorbents using additive manufacturing to increase the loading capacity as well as improve the preparation cost. In another study, Zhang et al. (2020) investigated the shape of zeolite monoliths to fabricate an ultrathin framework, thickness of 250 µm, by using this technique, which showed a significant carbon dioxide adsorption uptake in comparison with conventional zeolite.
Table 18.
Studies on additive manufacturing devoted to carbon dioxide adsorption
| Technique | Feed | Year | References |
|---|---|---|---|
| Direct ink writing | CO2, CH4, and N2 | 2017 | Couck et al. (2017) |
| Direct ink writing | CO2 and N2 | 2018 | Couck et al. (2018) |
| Direct ink writing | CO2 | 2019 | Regufe et al. (2019) |
| Selective laser sintering | CO2 | 2019 | Lahtinen et al. (2019) |
| Digital light processing | CO2 | 2020 | Zhang et al. (2020) |
| Direct ink writing | CO2 | 2020 | Lawson et al. (2020) |
| Direct ink writing | CO2, CH4, H2O, and N2 | 2020 | Grande et al. (2020) |
| Direct ink writing | CO2 | 2021 | Liu et al. (2021b) |
| Direct ink writing | CO2 and N2 | 2021 | Mendes et al. (2021) |
| Direct ink writing | CO2 and H2 | 2021 | Lawson and Rezaei (2021) |
CO2, carbon dioxide; CH4, methane; N2, nitrogen; H2O, water; and H2, hydrogen
Further, an interesting study was implemented by Steldinger et al. (2019), in which a carbonaceous adsorbent was printed and evaluated by electric swing adsorption cycles. Steldinger observed excellent thermal conductivity and higher carbon dioxide loading capacity in comparison with pellet carbon. The experiments proved shorter regeneration period with efficiency of 100%, which led to a significant decline in energy consumption (Steldinger et al. 2019). Steldinger concluded that carbon samples developed by additive manufacturing can be supreme potential carbon dioxide adsorbents through the electric swing adsorption processes (Steldinger et al. 2019).
Machine learning
Machine learning has been a fundamental element of many developing areas that represents a great capacity to obviate some of obstacles in traditional computing approaches for paradigm design, fault detection, cluster data, pattern recognition and optimization (Karimi et al. 2018c, 2021a, 2022a). The applications of machine learning owing to the robust computing power have increased in recent years, which is also true for carbon capture utilization and storage applications (Yan et al. 2021). In a simple word, machine learning detects a pattern/link between the key independent variables and the dependent ones of process (Karimi et al. 2021b, e), which finding these in the nonlinear systems contain partial differential equations using traditional approaches is a very tedious and time-consuming task.
Recently, different topologies related to the machine learning have emerged including artificial neural networks, adaptive neuro fuzzy inference system, support vector regression and genetic algorithm, which specific details regarding these models can be found in Karimi et al. (2018c, 2021a, b, e, 2022a). A comparison between the benefits and drawbacks of most common employed machine learning approaches in adsorption technology is described in Table 19 (Xu et al. 2022). Further, to better understand this concept, a simple architecture of multilayer feed-forward artificial neural network is displayed in Fig. 14.
Table 19.
Benefits and drawbacks of commonly employed machine learning approaches for the adsorption technology (Bansal et al. 2022; Xu et al. 2022)
| Models | Benefits | Drawbacks |
|---|---|---|
| Artificial Neural Networks | Minimum required input values to develop a model | Optimum iterations are required to be established for getting an accurate outcome |
| Highly robust approach to model any complex system with any degree of nonlinearity without any limitations concerning about the relation among input–output vector | Commonly a huge dataset is necessary to develop a precise model | |
| Getting an acceptable accuracy if developed systematically | Overfitting challenges accompanied with low ability for extrapolation issue | |
| Auto improvement for a provided set of data | Accuracy dependence on the number of layers, number of neurons, considered model as well as training algorithm | |
| Highly tolerance regarding the noisy data | ||
| Availability of wide ranges of the training algorithm | ||
| Adaptive Neuro Fuzzy Inference System | The ability for employing the benefits of ANNs and fuzzy logic, simultaneously | Enhancing the complexity of system coding regarding the number of bogus laws |
| Relatively, fast training algorithm | Requires too much computations attempts for developing an accurate approach | |
| Suitable for systems without basic knowledge about the system | Commonly getting an accurate mode needs a huge dataset | |
| Some beheld weaknesses concerning the extrapolation capacity | ||
| Support Vector Regression | Ability to be trained quickly | Model development requires a huge dataset |
| Simple theoretical background | Some weaknesses concerning the extrapolation capacity | |
| A broad range of choices owing to the availability of various kernel functions | Relatively highly time consuming for computation | |
| Low overfitting challenge with small decision elements in paradigm | Influenced by noisy databank | |
| High dimensionality with robust universal functionality | ||
| Genetic Algorithm | Double upgrading on the distinctive and incessant functions | Highly time consuming |
| More tolerance concerning the noise in comparison with ANN, ANFIS and SVR | Developing approach significantly relies on correctly implementation of the model | |
| Developing more initiative solutions for huge-sized space state | Commonly getting the optimum output or convergence is very arduous | |
| Proper for multi-objective systems |
ANNs, artificial neural networks; ANFIS, adaptive neuro fuzzy inference system; and SVR, support vector regression
Fig. 14.
An architecture of multilayer feed-forward artificial neural networks to develop a model for considered system with four independent variables and one output
Besides the previous mentioned benefits, machine learning also comprises saving time and cost, minimizing human intervention, as well as highly efficient for pattern recognition in multidimensional and multi-variety data (Jordan and Mitchell 2015; Karimi et al. 2018c, 2021a). On the other hand, the performance dependency on the size and the quality of dataset can limit its efficiency in the carbon capture area, because of lack of data availability to develop a robust intelligent approach. Further, the application of machine learning is sometimes influenced by limited physical constraints, interpretability, and the quality of data (Jordan and Mitchell 2015; Guidotti et al. 2018). Additionally in some cases, the model interpretation for an accurate paradigm through complex and nonlinear systems is arduous (Guidotti et al. 2018; Karimi et al. 2021e).
The application of this branch of artificial intelligence in the carbon capture and sequestration area is a novel concept and there is still a lot to investigate. However, the application of machine learning in adsorption technology can be classified in two main categories such as material science for developing and characterizing novel samples and engineering perspectives for process design and optimal assessment. Regarding the complexity of a proper adsorbent for efficient carbon dioxide capturing accompanied with almost unlimited pathways to develop the structures, correctly identifying a proper sorbent is a highly intricate task (Farmahini et al. 2018; Khurana and Farooq 2019; Subramanian Balashankar and Rajendran 2019; Burns et al. 2020).
On the grounds, the discovery and development of novel sorbents using conventional experimental methods require notable cost and time (Moghadam et al. 2019). Thus, recently, machine learning topologies received a significant attention for designing, charactering, and tuning the porous materials using supervised and unsupervised paradigms (Yazaydın et al. 2009; Wilmer et al. 2011; Rosen et al. 2020; Moosavi et al. 2020). Beside the synthesizing routes, other characters of adsorbents related to the textural properties such as selectivity, stability, synthesizability, working capacity, hydrophobicity and total life cycle costs have also been addressed by machine learning (Yazaydın et al. 2009; Wilmer et al. 2011; Collins et al. 2016; Boyd et al. 2019; Moosavi et al. 2020).
In this way, Collins et al. (2016) employed the machine learning potential for optimizing the favorable physical properties and functional characters of one of the metal–organic frameworks, MIL-47, to improve the loading capacity for post-combustion application that contributed to 400% improvement. Boyd et al. (2019) evaluated a huge database of adsorbent structures, around 300,000, to determine a synthesis protocol for hydrophobic adsorbaphore specifications. Further, Dureckova et al. (2019) considered gradient boosted trees regression topology for estimation the selectivity of carbon dioxide toward hydrogen accompanied with the working capacity of carbon dioxide in a broad range of samples. It was illustrated both geometric and chemical descriptors can be applied for these purposes (Dureckova et al. 2019).
In a similar attempt, the selectivity of carbon dioxide toward nitrogen and working capacity of the metal–organic frameworks were estimated using artificial neural networks by Burner et al. (2020). Additionally, Moghadam et al. (2019) investigated the potential of machine learning to estimate the mechanical properties of metal–organic frameworks. The accomplished studies in this area proved there is a huge amount of novel information, which can open the possibility of new windows for adsorption technology.
Designing and optimizing the adsorption processes is the second realm of machine learning. As already extensively discussed, cyclic adsorption processes are characterized regarding the desorption step, which normally these types of processes are described with a set of nonlinear partial differential equations derived from the mass, momentum and energy balances through the time and space until getting a cyclic steady state (Karimi et al. 2022b). On the other hand, the performance of process is relied on transient profiles of key variables including temperature, pressure, and composition, which concerning the high dimensionality of process, employing optimization approach is intricate and arduous.
To obviate such challenges, machine learning has been nominated as a superior candidate. The studies for designing and optimizing cyclic adsorption processes using this phenomenon can be classified in three classes (Yan et al. 2021). The first studies are related to determining the available pattern among the input and output variables of process in an optimized paradigm, which minimizes computational burdens using supervised topologies. The second class of investigations has been devoted to employing supervised machine learning regressions to specify the axial or time profiles in the developed process. Finally, in other attempts, supervised approaches have been considered to decrease the dimensionality of optimized cyclic adsorption process (Leperi et al. 2019; Hüllen et al. 2020; Pai et al. 2020; Vo et al. 2020; Yan et al. 2021).
For instance, Leperi et al. (Leperi et al. 2019) designed the main steps of a conventional pressure swing adsorption process for carbon dioxide capture from flue gas using neural networks. In this way, Pai et al. (2020) evaluated the potential of different artificial intelligent models for estimation the performance indicators and the bed profiles of a 4-step vacuum swing adsorption unit for carbon capture from flue gas. They reported that Gaussian process regression represented the most accurate results among different tested algorithms to predict the performance indicators, while artificial neural networks predicted the bed profiles properly (Pai et al. 2020).
Subraveti et al. (2019) utilized the artificial neural networks paradigm to optimize the recovery and purity of carbon dioxide in 8-step pressure swing adsorption unit for pre-combustion process. They also specified the relative importance of each decision variable on the objective functions (Subraveti et al. 2019).
Further, Vo et al. (2020) successfully employed feed-forward artificial neural networks approaches to minimize the production cost of an integrated steam methane reforming-based hydrogen plants containing membrane, cryogenic and pressure swing adsorption units to increase the hydrogen recovery and carbon dioxide capture from the tail gas. Additionally, Hüllen et al. (2020) evaluated three different artificial intelligent topologies to maximize the productivity of a temperature swing adsorption process for direct air capture system concerning the productivity, recovery, and energy consumption. It is notable that the developed machine learning algorithms are only trustworthy and valid in the training domain, and one must be cautious for the values outside the range.
Life cycle assessment
Life cycle assessment is a systematic and standardized topology for evaluating the environmental aspects of a product or process through their entire life cycle from the beginning to the end of life (Muralikrishna and Manickam 2017; Algren et al. 2021). Also, it can have a significant impact on developing tactical planning, designing the systems, improving the process outcomes as well as elevating the economic performance and public policy (Harwatt et al. 2017; Algren et al. 2021). Further, one of the major benefits of this topic is the proposing of various pathways for developing a process with lowest environmental burden (Muralikrishna and Manickam 2017; Harwatt et al. 2017).
On the grounds, life cycle assessment can supply a sustainable development with environmental, economic, and social evaluation, simultaneously (Algren et al. 2021). It should be considered that the different approaches of this methodology have already been specified by the International Organization for Standardization (ISO 2006a, b). Totally, life cycle assessment models are classified in three different classes including: process approach, input–output model, and hybrid topology (Algren et al. 2021).
Hence, process one is related to the general methods for modeling the production of a system as the sum of “unit processes”, which specifies the life cycle assessment-practitioner-defined subsystems (Junnila 2006). Accordingly, the practitioner of the life cycle assessment is necessary to determine and quantify the required inputs and any produced outputs. The input–output model as next approach is considered as a holistic estimation for economic evaluation and model validation in the life cycle assessment, also supplying data for the process approach (Junnila 2006; Muralikrishna and Manickam 2017; Algren et al. 2021). In addition, the hybrid topology is commonly a process approach that inputs–outputs data concerning the environmental impacts have been provided by input–output model (Muralikrishna and Manickam 2017).
As can be expected both process approach and input–output model of life cycle assessments possess some benefits and drawbacks. Totally, the flexibility of process approach is its main strength to evaluate product systems, but this approach can be expensive and time consuming in some cases (Shirzad et al. 2019b; Algren et al. 2021). On the other hand, the sectors specified in the related input–output table limit the resolution of input–output model that affects transactions between industries. However, the hybrid topology is considered to employ the benefits of process approach and input–output model of life cycle assessments (Muralikrishna and Manickam 2017; Algren et al. 2021).
Recently, life cycle assessment as a robust, adaptable and flexible tool has attracted a significant attention among existing and emerging technologies to specify the environmental hotspots of a product, service or process, which one of these areas is adsorption technology. In this way, attempts associated with this concept for adsorption technology can be categorized in three main classes such as devoted studies for evaluating the developed/synthesized sorbents for carbon capture (Wang et al. 2014b, 2021b; Wu et al. 2020; Nowrouzi et al. 2021; Heidari et al. 2022; Xia et al. 2022); the second studies have investigated the various aspects of adsorption process (Tang and You 2017; Kohlheb et al. 2021; Gonzalez-Olmos et al. 2022); the next ones are related to the evaluation of emerging technologies related to the carbon dioxide removal (Deutz and Bardow 2021; Terlouw et al. 2021a, b; Daniel et al. 2022; Leonzio et al. 2022).
In this way, Wang et al. (2014b) developed a life cycle assessment model for the evaluation of calcium oxide-based samples derived from the waste oyster shells blended with polymethyl methacrylate nanosphere scaffolds as a source of sorbent for carbon dioxide capture. They suggested that a remarkable carbon emission reduction accompanied with lesser human health and ecosystems impacts are accessible using waste in this way (Wang et al. 2014b). In other study, experimental results and a life cycle approach were combined to assess the carbon dioxide dual roles in food scraps-derived biochar activation to improve lead adsorption capacity (Wang et al. 2021b).
The environmental impacts and determining the hotspots of amines adsorbents for carbon dioxide capture were also investigated using life cycle assessment by Wu et al. (2020). In addition, the sustainability of activated carbon, amine-modified activated carbon, and graphene concerning the economic, environmental, and technical perspectives using this technique were analyzed by Heidari et al. (2022) for carbon capture and sequestration. A similar study was conducted by Nowrouzi et al. (2021) for two highly efficient synthesized carbon-based adsorbents including activated carbon and modified activated carbon for carbon dioxide adsorption.
The different synthesis techniques for developing ZIF-8, as one of the most promising metal–organic frameworks for carbon capture, was also assessed by this methodology to indicate the most sustainable method (Xia et al. 2022). Hu et al. (2021) employed this strategy for evaluating the energy load and resource depletion of 50 metal–organic frameworks concerning the synthesis procedure for carbon capture and storage. The eco-friendly fabrication of amide-based polymer adsorbents regarding the regeneration energy and selectivity for carbon dioxide capture was performed using the life cycle assessment by Fayemiwo et al. (Fayemiwo et al. 2019). Additionally, Tang and You (2017) assessed the environmental and economic aspects of municipal solid wastes incineration power plant with this topology for post-combustion carbon capture with zeolite 13X via two-stage pressure/vacuum swing adsorption processes.
In another attempt, the environment/economic aspects of biogas upgrading technology by pressure swing adsorption using life cycle assessment was performed by Kohlheb et al. (2021). Also, Gonzalez-Olmos compared the zeolite 13X and carbon molecular sieve for carbon capture from flue gas using vacuum pressure swing adsorption with this topology concerning the environmental impacts and the sustainability of process (Gonzalez-Olmos et al. 2022).
In this way, other researchers (Deutz and Bardow 2021) employed life cycle assessment for industrial direct air capture via temperature–vacuum swing adsorption process. Daniel et al. (2022) proposed and designed a novel approach for direct air capture integrated with a solid oxide electrolysis unit and assessed by this methodology. Further, Leonzio et al. (2022) investigated the environmental performance of three physisorbents, metal–organic frameworks, and two chemisorbents, amine functionalized sorbents, for direct air capture by developing a comprehensive life cycle assessment approach. Some of the interesting details concerning this topic related to the carbon dioxide removal technologies and adsorption processes can be found in Terlouw et al. (2021a, b).
Technology, commercialization and scale-up
This section details the history, current status and future challenges of scale-up and commercialization of adsorption technology for carbon capture. Indeed, carbon dioxide capture was firstly introduced to the industry in 1930s, for natural gas sweetening by separating carbon dioxide from methane using aqueous amines solutions (Kearns 2021). From that date, several other technologies have been evaluated including physical solvents, membranes, and physical adsorption for gas separation. The adsorption process using solid sorbents was firstly employed using pressure swing adsorption units for hydrogen purification in 1950s (Siqueira et al. 2017).
However, despite extensive efforts on adsorption technology, owing to the available challenges, all developed plants have been mainly limited in the bench and/or pilot scales (Akinola et al. 2022). Routinely, the progress of all technologies pass through the laboratory studies, bench scale, pilot scale and eventually, commercialization. To this end, the technology readiness level is considered as a general framework, which defines the maturity of one technology for deployment in the commercial scale (Kearns 2021).
In this way, the maturity of a technology is evaluated in a systematic and qualitative scaling way using technology readiness level in nine different levels (see Table 20) (Buchner et al. 2019; Kearns 2021; Zimmermann et al. 2022). Accordingly, technology readiness level of 1 represents the research step regarding the basic principles, observations, and initial concepts, while 9 corresponds with normal commercial service (Kearns 2021). On the grounds, carbon capture by liquid solvents in different processes is a technology, which has already completely reached to the commercial scale, while direct air capture as an emerging technology demonstrates level 6 (IEA 2022).
Table 20.
Definition of technology readiness level (TRL) for carbon capture and storage technologies (Wilcox et al. 2014; Kearns 2021; Zimmermann et al. 2022)
| Category | TRL | Definition | Description |
|---|---|---|---|
| Demonstration | 9 | Production | Process under normal operating condition over the full range of commercial service |
| 8 | Commissioning | Full-scale deployment in the final form for commercial applications | |
| 7 | Demonstration and full-scale engineering | Fully functional prototype demonstration in a relevant environment | |
| Development | 6 | Pilot trials | Pilot-scale experiments in a relevant environment |
| 5 | Detailed process development | Similar process validation in a relevant environment | |
| 4 | Preliminary process development | Process validation in the laboratory scale | |
| Research | 3 | Proof of concept | Proof of concept using analytical and experimental tests |
| 2 | Concept | Establishment of the application | |
| 1 | Idea | Initial idea and basic principles observed |
TRL, technology readiness level
To make adsorption technology competitive and cost effective one in comparison with other available techniques for carbon capture in large-scale applications, there are some barriers, which are required to be obviated for further proceeding. As already discussed in Sect. “Recent adsorbents for carbon dioxide capture”, a standard sorbent shall represent some key specifications such as stability concerning impurities, both chemicals and moisture, selectivity, fast kinetic, recyclability, high working capacity and rational synthesized cost (Wilcox et al. 2014).
Further, the thermal stability is one of other factors that requires to be taken into account according to the working temperature of sorbent that is classified in three different ranges including: higher than 400 °C, between 200 and 400 °C and lower than 200 °C, whereas for post-combustion carbon dioxide capture application, between 50 and 150 °C, this parameter may not be necessary (Wilcox et al. 2014).
However, deployment such sorbent in the industrial scale has been a big challenge of adsorption technology until lately, but recently, Svante Inc. in collaborating with Shimizu’ research group announced the successful synthesis of metal organic framework CALF-20 that addresses these challenges (Lin et al. 2021). CALF-20 showed a low manufacturing cost, selective over water and resistance to oxidation, as well as, it has the potential of carbon dioxide capturing up to 95% from industrial flue gas sources. Further, Svante Inc. in collaboration with BASF Co., have successfully scaled-up the CALF-20 in a green chemistry route to the industrial size (SvanteInc 2022). Up to now, large-scale production of such a sorbent for the gas separation industry has been one of the main challenges of commercialization of adsorption technology for carbon capture in the industrial scale.
Regarding the process development, carbon dioxide partial pressure and the scale of capturing are two other key elements that affect the cost and deployment of adsorption technology (Kearns 2021). Indeed, the partial pressure influences the required energy of plant for capturing and the size of process equipment. Hence, at the higher partial pressure the loading capacity is increased, the energy required for capturing reduces and molecules transfer more quickly from the gas source to the sorbent, which translates into smaller required capture equipment. Accordingly, all reduce the operating and capital costs (Kearns 2021).
Figure 15 illustrates the carbon capture cost as a function of carbon dioxide concentrations (IEA 2022). As can be found, direct air capture, because of the lowest carbon dioxide concentration, demonstrates the most expensive capturing processes. The other key factor that specifies the carbon capture cost is the scale of capturing (Akinola et al. 2022). Routinely, the capital cost of different industrial plants increases in a nonlinear relation with scale, including carbon dioxide capture plants. On the grounds, the capital cost is correlated by scale with the power of n, where n is in the range of 0.6–0.8. Thus, the cost of two different plants is related together by (Tribe and Alpine 1986):
| 1 |
Fig. 15.
Carbon capture cost of different processes as a function of carbon dioxide concentration, data from IEA for 2020 (IEA 2022). tCO2, tons of carbon dioxide; USD, United States Dollar; DAC, direct air capture; H2, hydrogen; CO2, carbon dioxide
As can be found, there is a significant difference between the cost of carbon capture pilot plant and an industrial scale for capturing tons of carbon dioxide per year. It should be considered, while this is a fact for all processes in various industries, and obviously carbon capture is not an exception, but regarding the technology readiness level of adsorption process for carbon dioxide capture and other mentioned challenges, this difference is more notable in this area (Kearns 2021). Table 21 represents an overview on different adsorption carbon capture processes regarding the technology readiness level assessment and key technology vendors in 2020 (Kearns 2021). Additionally, the world pioneer’s companies related to the CCUS technologies are illustrated in Table 22.
Table 21.
Adsorption carbon capture processes: technology readiness level assessment and key technology vendors in 2020 (Kearns 2021)
| Adsorption process | Key Vendors | Technology Readiness Level | Projects |
|---|---|---|---|
| Pressure swing adsorption/vacuum swing adsorption | Air Liquide, Air Products, UOP | 9 | Air products port Arthur |
| Temperature swing adsorption | Svante | 5–7 | Large pilot tests to feed studies for commercial plant |
| Enzyme catalyzed adsorption | CO2 solutions | 6 | Pilot demonstrations |
| Sorbent-enhanced water gas shift | ECN | 5 | Pilot tests, e.g., stepwise |
| Electrochemically mediated adsorption | R&D only | 1 | Lab test |
Table 22.
World pioneers companies related to carbon capture utilization and storage technologies
| Company | Description | Founded/ | Headquarter | References |
|---|---|---|---|---|
| Energy & Environmental Research Center | The EERC conducts research, development, demonstration, and commercialization activities related to zero-emissions coal conversion; carbon capture and sequestration; advanced air emission control technologies | 1951 | North Dakota, US | EERC (2022) |
| Wolf Carbon Solutions | Wolf Carbon Solutions conducts in constructing, owning, and operating carbon capture facilities and pipeline transportation systems related to decarbonizing industries | – | Denver, US | WolfCarbon |
| Chart Industries | Chart Industries, Inc. is a global manufacturer of engineered equipment primarily used for low-temperature and cryogenic gas storage applications regarding the CCS purposes | 1992 | Georgia, US | ChartInd (2022) |
| Svante | Svante offers commercially viable technology to capture industrial-scale carbon emissions from existing infrastructures | 2007 | Vancouver, Canada | SvanteInc (2022) |
| CarbonQuest | CarbonQuest provides technology and solutions to buildings owners seeking to decarbonize their buildings | 2019 | New York, US | CarbonQuest (2022) |
| OGCI Climate Investments LLP | OGCI Climate Investments LLP is an investment company, which invests in technologies and oil and gas sectors that represent the potential to decrease greenhouse gases emissions | 2014 | London, UK | OGCI (2022) |
| AirCapture | Direct air capture—negative emission technologies | 2018 | Berkeley, US | AirCapture (2022) |
| Carbon Engineering | Direct air capture—negative emission technologies | 2009 | British Columbia, Canada | CarbonEngineering (2022) |
| Climeworks | Direct air capture—negative emission technologies | 2009 | Zürich, Switzerland | Climeworks (2022) |
| Global Thermostat | Direct air capture—negative emission technologies | 2010 | Colorado, US | GlobalThermostat (2022) |
EERC, energy & environmental research center; CCS, carbon capture and storage
Regarding the necessity of decarbonization as well as the highly cost of carbon capture from dilute concentrations, owing to the thermodynamic laws, the US Department of Energy (DOE) has determined new aims to reduce the cost of carbon capture and to improve the state-of-the-art available carbon capture technologies including absorption, adsorption, membranes and cryogenic (Miller et al. 2016). In this way, second-generation technologies, which are expected to be deployed by 2025, are defined to decrease the capturing costs by 20 percent in comparison with current state-of-the-art technologies. Additionally, transformational technologies, which are available by 2030, are aimed to diminish the costs by 30 percent from the first kind of a technology. More details can be found in. (Kearns 2021).
Conclusion
The carbon dioxide emission and global warming resulted from anthropogenic activities accompanied with universal rising rate of energy consumption, specifically fossil fuels, have emerged as main challenges of mankind in twenty-first century. Accordingly, developing novel and more efficient technologies for carbon capture also finding renewable sources of energy have been considered as the key strategies to avoid an environmental calamity. Among various technologies to this end, adsorption technology with solid materials is one of the most attractive ones, which have already shown qualified potential for carbon capture and sequestration in double applications of post-combustion such as flue gas, and pre-combustion including biogas upgrading and natural gas sweeting processes.
On the grounds, this study aimed to highlight the current state of knowledge regarding the adsorption technology for carbon dioxide capture regarding clean energy and environmental protection. In-line with that, after a short discussion about carbon capture, climate change and the potential/prospect of biogas as a renewable source of energy, the key aspects of adsorption technology for such purposes alongside its recent advances were reviewed in a comprehensive way.
Accordingly, firstly, different carbon dioxide capture processes including post-combustion, pre-combustion and oxy-fuel combustion were introduced, then the pros and cons of different separation technologies were discussed by addressing the specific capital cost of each one. Further, different classes of sorbents were investigated in detail by referring to the most recent ones for carbon capture. The main adsorption techniques were also illustrated to determine how the loading capacity of various adsorbents are specified. Next, the most recent issues regarding the cyclic adsorption processes as the last step in developing adsorption technology were brought up.
To fully understand the novel topics in this area, direct air capture, machine learning, life cycle assessment, challenges of commercialization and scale-up in adsorption technology were also critically reviewed to pave the path for future studies in this area.
Acknowledgements
This study was funded by LA/P/0045/2020 (ALiCE), UIDB/50020/2020, and UIDP/50020/2020 (LSRE-LCM), funded by national funds through FCT/MCTES (PIDDAC). Also, this work received financial support through national funds through FCT/MCTES (PIDDAC), CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2020). Mohsen Karimi also acknowledges PhD research grant awarded by Foundation of Science and Technology of Portugal (FCT) under SFRH/BD/140550/2018 project.
Author contributions
MK and MS: performing the literature search and data analysis, writing and editing the manuscript; organizing the figures and Tables; JACS: conceptualization, reviewing and editing, supervision; AER: conceptualization, reviewing and editing, supervision.
Declarations
Conflict of interest
Authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd AA, Othman MR, Kim J. A review on application of activated carbons for carbon dioxide capture: present performance, preparation, and surface modification for further improvement. Environ Sci Pollut Res. 2021;28(32):43329–43364. doi: 10.1007/S11356-021-15121-9. [DOI] [PubMed] [Google Scholar]
- AbdulKareem FA, Shariff AM, Ullah S, et al. Adsorption performance of 5A molecular sieve zeolite in water vapor–binary gas environment: experimental and modeling evaluation. J Ind Eng Chem. 2018;64:173–187. doi: 10.1016/J.JIEC.2018.03.014. [DOI] [Google Scholar]
- AGGI (2022) Climate change: annual greenhouse gas index, https://www.climate.gov/news-features/understanding-climate/climate-change-annual-greenhouse-gas-index
- Aghel B, Behaein S, Wongwises S, Shadloo MS. A review of recent progress in biogas upgrading: with emphasis on carbon capture. Biomass Bioenergy. 2022;160:106422. doi: 10.1016/J.BIOMBIOE.2022.106422. [DOI] [Google Scholar]
- Ahmed SF, Mofijur M, Tarannum K, et al. Biogas upgrading, economy and utilization: a review. Environ Chem Lett. 2021;19(6):4137–4164. doi: 10.1007/S10311-021-01292-X. [DOI] [Google Scholar]
- Ahn H, Lee CHA, Seo B, et al. Backfill cycle of a layered bed H2 PSA process. Adsorption. 1999;5(4):419–433. doi: 10.1023/A:1008973118852. [DOI] [Google Scholar]
- AirCapture (2022) https://www.aircapture.com/
- Akinola TE, Bonilla Prado PL, Wang M. Experimental studies, molecular simulation and process modelling\simulation of adsorption-based post-combustion carbon capture for power plants: a state-of-the-art review. Appl Energy. 2022;317:119156. doi: 10.1016/J.APENERGY.2022.119156. [DOI] [Google Scholar]
- Al Mesfer MK, Danish M, Ali IH, Khan MI. Adsorption behavior of molecular sieve 3 Å and silica gel for CO2 separation: equilibrium, breakthrough and mass transfer zone. Heat Mass Transf waerme Stoffuebertrag. 2020;56:3243–3259. doi: 10.1007/S00231-020-02923-9/FIGURES/16. [DOI] [Google Scholar]
- Alberici S, Grimme W, Toop G (2022) Biomethane production potentials in the EU, Feasibility of REPowerEU 2030 targets, production potentials in the Member Biomethane production potentials in the EU
- Algren M, Fisher W, Landis AE (2021) Machine learning in life cycle assessment. In: Data science applied to sustainability analysis. Elsevier, pp 167–190
- Álvarez-Gutiérrez N, García S, Gil MV, et al. Towards bio-upgrading of biogas: biomass waste-based adsorbents. Energy Procedia. 2014;63:6527–6533. doi: 10.1016/J.EGYPRO.2014.11.688. [DOI] [Google Scholar]
- An L, Liu S, Wang L, et al. Novel nitrogen-doped porous carbons derived from graphene for effective CO2 capture. Ind Eng Chem Res. 2019;58:3349–3358. doi: 10.1021/acs.iecr.8b06122. [DOI] [Google Scholar]
- Arvind R, Farooq S, Ruthven DM. Analysis of a piston PSA process for air separation. Chem Eng Sci. 2002;57:419–433. doi: 10.1016/S0009-2509(01)00374-8. [DOI] [Google Scholar]
- ASTM I (2015) ASTM Int
- Bae YS, Liu J, Wilmer CE, et al. The effect of pyridine modification of Ni–DOBDC on CO2 capture under humid conditions. Chem Commun. 2014;50:3296–3298. doi: 10.1039/C3CC44954H. [DOI] [PubMed] [Google Scholar]
- Baena-Moreno FM, Rodríguez-Galán M, Vega F, et al. Biogas upgrading by cryogenic techniques. Environ Chem Lett. 2019;17:1251–1261. doi: 10.1007/S10311-019-00872-2/FIGURES/10. [DOI] [Google Scholar]
- Bansal M, Goyal A, Choudhary A. A comparative analysis of K-nearest neighbor, genetic, support vector machine, decision tree, and long short term memory algorithms in machine learning. Decis Anal J. 2022;3:100071. doi: 10.1016/J.DAJOUR.2022.100071. [DOI] [Google Scholar]
- Bao Z, Yu L, Ren Q, et al. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J Colloid Interface Sci. 2011;353:549–556. doi: 10.1016/J.JCIS.2010.09.065. [DOI] [PubMed] [Google Scholar]
- Barthomeuf D. Conjugate acid–base pairs in zeolites. J Phys Chem. 2002;88:42–45. doi: 10.1021/j150645a010. [DOI] [Google Scholar]
- Bartle KD, Myers P. History of gas chromatography. TrAC Trends Anal Chem. 2002;21:547–557. doi: 10.1016/S0165-9936(02)00806-3. [DOI] [Google Scholar]
- Basmadjian D, Pogorski A (1963) Process for the separation of gases by adsorption. US Patent 3,279,153
- Bastos-Neto M, Möller A, Staudt R, et al. Breakthrough curves of methane at high pressures for H2 purification processes. Chem Ing Tech. 2011;83:183–190. doi: 10.1002/CITE.201000155. [DOI] [Google Scholar]
- Ben-Mansour R, Habib MA, Bamidele OE, et al. Carbon capture by physical adsorption: materials, experimental investigations and numerical modeling and simulations—a review. Appl Energy. 2016;161:225–255. doi: 10.1016/J.APENERGY.2015.10.011. [DOI] [Google Scholar]
- Bering BP, Serpinskij VV. Volumetric-Gravimetric method for determination of adsorption of gas mixtures (Russ.) Dokl Akad Nauk SSSR. 1953;5:811–814. [Google Scholar]
- Boyd PG, Chidambaram A, García-Díez E, et al. Data-driven design of metal–organic frameworks for wet flue gas CO2 capture. Nature. 2019;576(7786):253–256. doi: 10.1038/s41586-019-1798-7. [DOI] [PubMed] [Google Scholar]
- Broom D, Talu O, Benham M. Integral mass balance (IMB) method for measuring multicomponent gas adsorption equilibria in nanoporous materials. Ind Eng Chem Res. 2020;59:20478–20491. doi: 10.1021/acs.iecr.0c04162. [DOI] [Google Scholar]
- Buchner GA, Stepputat KJ, Zimmermann AW, Schomäcker R. Specifying technology readiness levels for the chemical industry. Ind Eng Chem Res. 2019;58:6957–6969. doi: 10.1021/acs.iecr.8b05693. [DOI] [Google Scholar]
- Bülow M. Adsorption of gas mixtures: comment on “Opening the Toolbox: 18 experimental techniques for measurement of mixed gas adsorption”. Ind Eng Chem Res. 2022;61:5009–5010. doi: 10.1021/acs.iecr.2c00618. [DOI] [Google Scholar]
- Burner J, Schwiedrzik L, Krykunov M, et al. High-performing deep learning regression models for predicting low-pressure CO2 adsorption properties of metal–organic frameworks. J Phys Chem C. 2020;124:27996–28005. doi: 10.1021/acs.jpcc.0c06334. [DOI] [Google Scholar]
- Burns DT, Nagesh Pai K, Gokul Subraveti S, et al. Prediction of MOF performance in vacuum swing adsorption systems for postcombustion CO2 capture based on integrated molecular simulations, process optimizations, and machine learning models. Environ Sci Technol. 2020;54:4536–4544. doi: 10.1021/acs.est.9b07407. [DOI] [PubMed] [Google Scholar]
- Byung Yoon K. Electron- and charge-transfer reactions within zeolites. Chem Rev. 2002;93:321–339. doi: 10.1021/cr00017a015. [DOI] [Google Scholar]
- Canevesi RL, Andreassen K, Silva E, et al. Pressure swing adsorption for biogas upgrading with carbon molecular sieve. Ind Eng Chem Res. 2018;57:8057–8067. doi: 10.1021/acs.iecr.8b00996. [DOI] [Google Scholar]
- Caplow M. Kinetics of carbamate formation and breakdown. J Am Chem Soc. 1968;90:6795–6803. doi: 10.1021/ja01026a041. [DOI] [Google Scholar]
- CarbonEngineering (2022) http://carbonengineering.com/
- CarbonQuest (2022) https://carbonquest.com
- Chakraborty A, Maji TK. Mg-MOF-74@SBA-15 hybrids: synthesis, characterization, and adsorption properties. APL Mater. 2014;2:124107. doi: 10.1063/1.4902816. [DOI] [Google Scholar]
- ChartInd (2022) http://www.chartindustries.com/
- Chen Y, Lv D, Wu J, et al. A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem Eng J. 2017;308:1065–1072. doi: 10.1016/J.CEJ.2016.09.138. [DOI] [Google Scholar]
- Chen C, Feng N, Guo Q, et al. Surface engineering of a chromium metal–organic framework with bifunctional ionic liquids for selective CO2 adsorption: synergistic effect between multiple active sites. J Colloid Interface Sci. 2018;521:91–101. doi: 10.1016/J.JCIS.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wu H, Liu Z, et al. Liquid-assisted mechanochemical synthesis of copper based MOF-505 for the separation of CO2 over CH4 or N2. Ind Eng Chem Res. 2018;57:703–709. doi: 10.1021/acs.iecr.7b03712. [DOI] [Google Scholar]
- Chen C, Jiang Q, Xu H, Lin Z. Highly efficient synthesis of a moisture-stable nitrogen-abundant metal–organic framework (MOF) for large-scale CO2 capture. Ind Eng Chem Res. 2019;58:1773–1777. doi: 10.1021/acs.iecr.8b05239. [DOI] [Google Scholar]
- Chen G, Wang F, Wang S, et al. Facile fabrication of copper oxide modified activated carbon composite for efficient CO2 adsorption. Korean J Chem Eng. 2021;38(1):46–54. doi: 10.1007/S11814-020-0684-1. [DOI] [Google Scholar]
- Choi S, Drese JH, Jones CW. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem. 2009;2:796–854. doi: 10.1002/CSSC.200900036. [DOI] [PubMed] [Google Scholar]
- Christopher J. CO2 capture from dilute gases as a component of modern global carbon management. Annu Rev Chem Biomol Eng. 2011;2:31–52. doi: 10.1146/ANNUREV-CHEMBIOENG-061010-114252. [DOI] [PubMed] [Google Scholar]
- Clausse M, Merel J, Meunier F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption. Int J Greenhouse Gas Control. 2011;5:1206–1213. doi: 10.1016/J.IJGGC.2011.05.036. [DOI] [Google Scholar]
- Climeworks (2022) https://climeworks.com
- Collins SP, Daff TD, Piotrkowski SS, Woo TK. Materials design by evolutionary optimization of functional groups in metal–organic frameworks. Sci Adv. 2016 doi: 10.1126/SCIADV.1600954/SUPPL_FILE/1600954_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Sotelo JL, Calleja G, Marrón C. Adsorption of binary and ternary hydrocarbon gas mixtures on activated carbon: experimental determination and theoretical prediction of the ternary equilibrium data. AIChE J. 1981;27:5–12. doi: 10.1002/AIC.690270103. [DOI] [Google Scholar]
- Côté AP, Benin AI, Ockwig NW, et al. Chemistry: porous, crystalline, covalent organic frameworks. Science. 2005;310:1166–1170. doi: 10.1126/SCIENCE.1120411/SUPPL_FILE/COTE.SOM.PDF. [DOI] [PubMed] [Google Scholar]
- Couck S, Lefevere J, Mullens S, et al. CO2, CH4 and N2 separation with a 3DFD-printed ZSM-5 monolith. Chem Eng J. 2017;308:719–726. doi: 10.1016/J.CEJ.2016.09.046. [DOI] [Google Scholar]
- Couck S, Cousin-Saint-Remi J, van der Perre S, et al. 3D-printed SAPO-34 monoliths for gas separation. Microporous Mesoporous Mater. 2018;255:185–191. doi: 10.1016/J.MICROMESO.2017.07.014. [DOI] [Google Scholar]
- Council NR (2015) Climate intervention: carbon dioxide removal and reliable sequestration. In: Climate intervention: carbon dioxide removal and reliable sequestration, pp 1–154. 10.17226/18805
- Danaei Kenarsari S, Fan M, Jiang G, et al. Use of a robust and inexpensive nanoporous TiO2 for pre-combustion CO2 separation. Energy Fuels. 2013;27:6938–6947. doi: 10.1021/ef4019004. [DOI] [Google Scholar]
- Danckwerts PV. The reaction of CO2 with ethanolamines. Chem Eng Sci. 1979;34:443–446. doi: 10.1016/0009-2509(79)85087-3. [DOI] [Google Scholar]
- Daniel T, Masini A, Milne C, et al. Techno-economic analysis of direct air carbon capture with CO2 utilisation. Carbon Capture Sci Technol. 2022;2:100025. doi: 10.1016/J.CCST.2021.100025. [DOI] [Google Scholar]
- de Montgareuil PG, Daniel D (1958) Process for separating a binary gaseous mixture by adsorption. US Patent 3155468A. 155
- de Weireld G, Belmabkhout Y, Frère M. Buoyancy effect correction on high pressure pure gas adsorption gravimetric measurements. Ann Chim Sci Mater. 2005;30:411–423. doi: 10.3166/acsm.30.411-423. [DOI] [Google Scholar]
- Deng S, Hu B, Chen T, et al. Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption. 2015;21:125–133. doi: 10.1007/S10450-015-9655-Y/FIGURES/8. [DOI] [Google Scholar]
- Deutz S, Bardow A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat Energy. 2021;6(2):203–213. doi: 10.1038/s41560-020-00771-9. [DOI] [Google Scholar]
- Didas S, Choi S, Chaikittisilp W, Jones CW. Amine-oxide hybrid materials for CO2 capture from ambient air. Acc Chem Res. 2015;48:2680–2687. doi: 10.1021/acs.accounts.5b00284. [DOI] [PubMed] [Google Scholar]
- Ding N, Li H, Feng X, et al. Partitioning MOF-5 into confined and hydrophobic compartments for carbon capture under humid conditions. J Am Chem Soc. 2016;138:10100–10103. doi: 10.1021/jacs.6b06051. [DOI] [PubMed] [Google Scholar]
- Dobladez JAD, Maté VIÁ, Torrellas SÁ, Larriba M. Separation of the propane propylene mixture with high recovery by a dual PSA process. Comput Chem Eng. 2020;136:106717. doi: 10.1016/J.COMPCHEMENG.2019.106717. [DOI] [Google Scholar]
- Dong K, Jiang H, Sun R, Dong X. Driving forces and mitigation potential of global CO2 emissions from 1980 through 2030: evidence from countries with different income levels. Sci Total Environ. 2019;649:335–343. doi: 10.1016/J.SCITOTENV.2018.08.326. [DOI] [PubMed] [Google Scholar]
- Dreisbach F, Staudt R, Keller JU. High pressure adsorption data of methane, nitrogen, carbon dioxide and their binary and ternary mixtures on activated carbon. Adsorption. 1999;5(3):215–227. doi: 10.1023/A:1008914703884. [DOI] [Google Scholar]
- Dureckova H, Krykunov M, Zein Aghaji M, Woo TK. Robust machine learning models for predicting high CO2 working capacity and CO2/H2 selectivity of gas adsorption in metal organic frameworks for precombustion carbon capture. J Phys Chem C. 2019;123:4133–4139. doi: 10.1021/acs.jpcc.8b10644. [DOI] [Google Scholar]
- EBA (2021) EBA Activity Report 2021 | European Biogas Association, https://www.europeanbiogas.eu/eba-activity-report-2021/
- EERC (2022) Energy & Environmental Research Center, www.undeerc.org.
- Eisenberger P (2012) Carbon dioxide capture/regeneration structures, and techniques. US Patent 8163066B2
- Eisenberger P, Chichilnisky G (2007) System and method for removing carbon dioxide from an atmosphere and global thermostat using the same. US Patent 20080289319A1
- European Commission . European Commission Recommendation on speeding up permit-granting procedures for renewable energy projects and facilitating Power Purchase Agreements. Brussels: European Commission; 2022. [Google Scholar]
- European Commission (2022b) Renewable energy targets. https://energy.ec.europa.eu
- Evans HA, Mullangi D, Deng Z, et al. Aluminum formate, Al(HCOO)3: an earth-abundant, scalable, and highly selective material for CO2 capture. Sci Adv. 2022;8:eade1473. doi: 10.1126/SCIADV.ADE1473/SUPPL_FILE/SCIADV.ADE1473_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakher S, Imqam A. A review of carbon dioxide adsorption to unconventional shale rocks methodology, measurement, and calculation. SN Appl Sci. 2020;2:1–14. doi: 10.1007/S42452-019-1810-8/TABLES/6. [DOI] [Google Scholar]
- FAO (2022) Statistical yearbook. World food and agriculture, www.fao.org
- Farghali M, Osman AI, Umetsu K, Rooney DW. Integration of biogas systems into a carbon zero and hydrogen economy: a review. Environ Chem Lett. 2022;20(5):2853–2927. doi: 10.1007/S10311-022-01468-Z. [DOI] [Google Scholar]
- Farmahini A, Krishnamurthy S, Friedrich D, et al. From crystal to adsorption column: challenges in multiscale computational screening of materials for adsorption separation processes. Ind Eng Chem Res. 2018;57:15491–15511. doi: 10.1021/acs.iecr.8b03065. [DOI] [Google Scholar]
- Fasihi M, Efimova O, Breyer C. Techno-economic assessment of CO2 direct air capture plants. J Clean Prod. 2019;224:957–980. doi: 10.1016/J.JCLEPRO.2019.03.086. [DOI] [Google Scholar]
- Fayemiwo KA, Chiarasumran N, Nabavi SA, et al. Eco-friendly fabrication of a highly selective amide-based polymer for CO2 capture. Ind Eng Chem Res. 2019;58:18160–18167. doi: 10.1021/acs.iecr.9b02347. [DOI] [Google Scholar]
- Feng X, Ding X, Jiang D. Covalent organic frameworks. Chem Soc Rev. 2012;41:6010–6022. doi: 10.1039/C2CS35157A. [DOI] [PubMed] [Google Scholar]
- Ferreira AFP, Ribeiro AM, Kulaç S, Rodrigues AE. Methane purification by adsorptive processes on MIL-53(Al) Chem Eng Sci. 2015;124:79–95. doi: 10.1016/J.CES.2014.06.014. [DOI] [Google Scholar]
- Fuderer A (1983) Pressure swing adsorption with intermediate product recovery. US Patent 4512780A
- Furukawa H, Yaghi OM. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J Am Chem Soc. 2009;131:8875–8883. doi: 10.1021/ja9015765. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. The chemistry and applications of metal–organic frameworks. Science. 2013 doi: 10.1126/SCIENCE.1230444/SUPPL_FILE/FURUKAWA.SM.CORRECTED.PDF. [DOI] [PubMed] [Google Scholar]
- Fuss S, Lamb WF, Callaghan MW, et al. Negative emissions—part 2: costs, potentials and side effects. Environ Res Lett. 2018;13:063002. doi: 10.1088/1748-9326/AABF9F. [DOI] [Google Scholar]
- Gaikwad S, Kim Y, Gaikwad R, Han S. Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework. J Environ Chem Eng. 2021;9:105523. doi: 10.1016/J.JECE.2021.105523. [DOI] [Google Scholar]
- Gebald C, Wurzbacher JA, Borgschulte A, et al. Single-component and binary CO2 and H2O adsorption of amine-functionalized cellulose. Environ Sci Technol. 2014;48:2497–2504. doi: 10.1021/es404430g. [DOI] [PubMed] [Google Scholar]
- Ghanbari T, Abnisa F, Wan Daud WMA. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci Total Environ. 2020;707:135090. doi: 10.1016/J.SCITOTENV.2019.135090. [DOI] [PubMed] [Google Scholar]
- GlobalThermostat (2022) https://Globalthermostat.com
- Glonek K, Wróblewska A, Makuch E, et al. Oxidation of limonene using activated carbon modified in dielectric barrier discharge plasma. Appl Surf Sci. 2017;420:873–881. doi: 10.1016/J.APSUSC.2017.05.136. [DOI] [Google Scholar]
- Gole B, Stepanenko V, Rager S, et al. Microtubular self-assembly of covalent organic frameworks. Angew Chem Int Ed. 2018;57:846–850. doi: 10.1002/ANIE.201708526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González AS, Plaza MG, Rubiera F, Pevida C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem Eng J. 2013;230:456–465. doi: 10.1016/J.CEJ.2013.06.118. [DOI] [Google Scholar]
- Gonzalez-Olmos R, Gutierrez-Ortega A, Sempere J, Nomen R. Zeolite versus carbon adsorbents in carbon capture: a comparison from an operational and life cycle perspective. J CO2 Util. 2022;55:101791. doi: 10.1016/J.JCOU.2021.101791. [DOI] [Google Scholar]
- Grande CA. Advances in pressure swing adsorption for gas separation. ISRN Chem Eng. 2012;2012:1–13. doi: 10.5402/2012/982934. [DOI] [Google Scholar]
- Grande CA, Ribeiro RPL, Oliveira ELG, Rodrigues AE. Electric swing adsorption as emerging CO2 capture technique. Energy Procedia. 2009;1:1219–1225. doi: 10.1016/J.EGYPRO.2009.01.160. [DOI] [Google Scholar]
- Grande CA, Blom R, Middelkoop V, et al. Multiscale investigation of adsorption properties of novel 3D printed UTSA-16 structures. Chem Eng J. 2020;402:126166. doi: 10.1016/J.CEJ.2020.126166. [DOI] [Google Scholar]
- Gu C, Liu D, Huang W, et al. Synthesis of covalent triazine-based frameworks with high CO2 adsorption and selectivity. Polym Chem. 2015;6:7410–7417. doi: 10.1039/C5PY01090J. [DOI] [Google Scholar]
- Guidotti R, Monreale A, Ruggieri S, et al. A survey of methods for explaining black box models. ACM Comput Surv (CSUR) 2018 doi: 10.1145/3236009. [DOI] [Google Scholar]
- Hamon L, Chenoy L, de Weireld G. Determination of absolute gas adsorption isotherms: simple method based on the potential theory for buoyancy effect correction of pure gas and gas mixtures adsorption. Adsorption. 2014;20:397–408. doi: 10.1007/S10450-013-9579-3/TABLES/3. [DOI] [Google Scholar]
- Hao W, Björkman E, Lilliestråle M, Hedin N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl Energy. 2013;112:526–532. doi: 10.1016/J.APENERGY.2013.02.028. [DOI] [Google Scholar]
- Harwatt H, Sabaté J, Eshel G, et al. Substituting beans for beef as a contribution toward US climate change targets. Clim Change. 2017;143(1):261–270. doi: 10.1007/S10584-017-1969-1. [DOI] [Google Scholar]
- He B, Sadiq MM, Batten MP, et al. Continuous flow synthesis of a Zr magnetic framework composite for post-combustion CO2 capture. Chem A Eur J. 2019;25:13184–13188. doi: 10.1002/CHEM.201902560. [DOI] [PubMed] [Google Scholar]
- He X, Liu J, Jiang Y, et al. ‘Useful’ template synthesis of N-doped acicular hollow porous carbon/carbon-nanotubes for enhanced capture and selectivity of CO2. Chem Eng J. 2019;361:278–285. doi: 10.1016/J.CEJ.2018.12.031. [DOI] [Google Scholar]
- Heidari A, Boleydei H, Rohani A, et al. Integrating life cycle assessment and life cycle costing using TOPSIS to select sustainable biomass-based -carbonaceous adsorbents for CO2 capture. J Clean Prod. 2022;357:131968. doi: 10.1016/J.JCLEPRO.2022.131968. [DOI] [Google Scholar]
- Hemalatha P, Bhagiyalakshmi M, Ganesh M, et al. Role of ceria in CO2 adsorption on NaZSM-5 synthesized using rice husk ash. J Ind Eng Chem. 2012;18:260–265. doi: 10.1016/J.JIEC.2011.11.046. [DOI] [Google Scholar]
- Henrique A, Karimi M, Silva JAC, Rodrigues AE. Analyses of adsorption behavior of CO2, CH4, and N2 on different types of BETA zeolites. Chem Eng Technol. 2019;42:327–342. doi: 10.1002/CEAT.201800386. [DOI] [Google Scholar]
- Hiyoshi N, Yogo K, Yashima T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005;84:357–365. doi: 10.1016/J.MICROMESO.2005.06.010. [DOI] [Google Scholar]
- Ho M, Allinson GW, Wiley DE. Reducing the cost of CO2 capture from flue gases using pressure swing adsorption. Ind Eng Chem Res. 2008;47:4883–4890. doi: 10.1021/ie070831e. [DOI] [Google Scholar]
- House KZ, Baclig AC, Ranjan M, et al. Economic and energetic analysis of capturing CO2 from ambient air. Proc Natl Acad Sci USA. 2011;108:20428–20433. doi: 10.1073/PNAS.1012253108/SUPPL_FILE/APPENDIX.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovestadt M, Friebe S, Helmich L, et al. Continuous separation of light olefin/paraffin mixtures on ZIF-4 by pressure swing adsorption and membrane permeation. Molecules. 2018;23(889 23):889. doi: 10.3390/MOLECULES23040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P-F, Xuan Law Z, Lin C-H, Tsai D-H. Understanding solvothermal growth of metal–organic framework colloids for CO2 capture applications. Langmuir. 2022;38:4415–4424. doi: 10.1021/acs.langmuir.2c00165. [DOI] [PubMed] [Google Scholar]
- Hu Y, Verdegaal WM, Yu SH, Jiang HL. Alkylamine-tethered stable metal–organic framework for CO2 capture from flue gas. Chemsuschem. 2014;7:734–737. doi: 10.1002/CSSC.201301163. [DOI] [PubMed] [Google Scholar]
- Hu J, Gu X, Lin L-C, Bakshi RB. Toward sustainable metal–organic frameworks for post-combustion carbon capture by life cycle assessment and molecular simulation. ACS Sustain Chem Eng. 2021;9:12132–12141. doi: 10.1021/acssuschemeng.1c03473. [DOI] [Google Scholar]
- Huang L, Cao G. 2D squaraine-bridged covalent organic polymers with promising CO2 storage and separation properties. ChemistrySelect. 2016;1:533–538. doi: 10.1002/SLCT.201600093. [DOI] [Google Scholar]
- Huang L, Wang H, Chen J, et al. Synthesis, morphology control, and properties of porous metal–organic coordination polymers. Microporous Mesoporous Mater. 2003;58:105–114. doi: 10.1016/S1387-1811(02)00609-1. [DOI] [Google Scholar]
- Huang G, Isfahani AP, Muchtar A, et al. Pebax/ionic liquid modified graphene oxide mixed matrix membranes for enhanced CO2 capture. J Memb Sci. 2018;565:370–379. doi: 10.1016/J.MEMSCI.2018.08.026. [DOI] [Google Scholar]
- Hüllen G, Zhai J, Kim SH, et al. Managing uncertainty in data-driven simulation-based optimization. Comput Chem Eng. 2020;136:106519. doi: 10.1016/J.COMPCHEMENG.2019.106519. [DOI] [Google Scholar]
- Humphrey JL, Keller GE. Separation process technology. New York: McGraw-Hill; 1997. [Google Scholar]
- IEA (2022) International Energy Agency (IEA). https://www.iea.org/
- Inui T, Okugawa Y, Yasuda M. Relationship between properties of various zeolites and their carbon dioxide adsorption behaviors in pressure swing adsorption operation. Ind Eng Chem Res. 2002;27:1103–1109. doi: 10.1021/ie00079a003. [DOI] [Google Scholar]
- IPCC . Climate Change 2022: impacts, adaptation and vulnerability. Contribution of Working Group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge, UK and New York: Cambridge University Press; 2022. p. 3056. [Google Scholar]
- ISO (2006a) Environmental management: life cycle assessment; Principles and framework. ISO 14040
- ISO (2006b) Environmental management: life cycle assessment; Requirements and guidelines. ISO 14040
- Jahromi PE, Fatemi S, Vatani A, et al. Purification of helium from a cryogenic natural gas nitrogen rejection unit by pressure swing adsorption. Sep Purif Technol. 2018;193:91–102. doi: 10.1016/J.SEPPUR.2017.11.002. [DOI] [Google Scholar]
- James AT, Martin AJ. Gas–liquid partition chromatography: the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem J. 1952;50:679–690. doi: 10.1042/BJ0500679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianguo X, Rarig D, Cook T, et al (2001) Pressure swing adsorption process with reduced pressure equalization time. US Patent 20030015091A1
- Jin C, Sun J, Chen Y, et al. Sawdust wastes-derived porous carbons for CO2 adsorption. Part 1. Optimization preparation via orthogonal experiment. Sep Purif Technol. 2021;276:119270. doi: 10.1016/J.SEPPUR.2021.119270. [DOI] [Google Scholar]
- Jordan M, Mitchell T. Machine learning: trends, perspectives, and prospects. Science. 2015;349:255–260. doi: 10.1126/SCIENCE.AAA8415. [DOI] [PubMed] [Google Scholar]
- Junnila S. Empirical comparison of process and economic input–output life cycle assessment in service industries. Environ Sci Technol. 2006;40:7070–7076. doi: 10.1021/es0611902. [DOI] [PubMed] [Google Scholar]
- Kahle H (1950) US Patent 2661808A
- Kahveci Z, Islamoglu T, Shar GA, et al. Targeted synthesis of a mesoporous triptycene-derived covalent organic framework. CrystEngComm. 2013;15:1524–1527. doi: 10.1039/C2CE26487K. [DOI] [Google Scholar]
- Kapoor A, Yang RT. Roll-up in fixed-bed, multicomponent adsorption under pore-diffusion limitation. AIChE J. 1987;33:1215–1217. doi: 10.1002/AIC.690330717. [DOI] [Google Scholar]
- Karimi M, Rahimpour MR, Rafiei R, et al. Reducing environmental problems and increasing saving energy by proposing new configuration for moving bed thermally coupled reactors. J Nat Gas Sci Eng. 2014;17:136–150. doi: 10.1016/J.JNGSE.2014.01.007. [DOI] [Google Scholar]
- Karimi M, Rahimpour MR, Iranshahi D. Enhanced BTX production in refineries with sulfur dioxide oxidation by thermal integrated model. Chem Eng Technol. 2018;41:1746–1758. doi: 10.1002/CEAT.201700289. [DOI] [Google Scholar]
- Karimi M, Silva JAC, Gonçalves CNDP, et al. CO2 capture in chemically and thermally modified activated carbons using breakthrough measurements: experimental and modeling study. Ind Eng Chem Res. 2018;57:11154–11166. doi: 10.1021/ACS.IECR.8B00953. [DOI] [Google Scholar]
- Karimi M, Vaferi B, Hosseini SH, Rasteh M. Designing an efficient artificial intelligent approach for estimation of hydrodynamic characteristics of tapered fluidized bed from its design and operating parameters. Ind Eng Chem Res. 2018;57:259–267. doi: 10.1021/ACS.IECR.7B02869. [DOI] [Google Scholar]
- Karimi M, Diaz de Tuesta JL, Carmem CN, et al. Compost from municipal solid wastes as a source of biochar for CO2 capture. Chem Eng Technol. 2020;43:1336–1349. doi: 10.1002/CEAT.201900108. [DOI] [Google Scholar]
- Karimi M, Zafanelli LFAS, Almeida JPP, et al. Novel insights into activated carbon derived from municipal solid waste for CO2 uptake: synthesis, adsorption isotherms and scale-up. J Environ Chem Eng. 2020 doi: 10.1016/J.JECE.2020.104069. [DOI] [Google Scholar]
- Karimi M, Aminzadehsarikhanbeglou E, Vaferi B. Robust intelligent topology for estimation of heat capacity of biochar pyrolysis residues. Measurement. 2021 doi: 10.1016/J.MEASUREMENT.2021.109857. [DOI] [Google Scholar]
- Karimi M, Hosin Alibak A, Mehdi Seyed Alizadeh S, et al. Intelligent modeling for considering the effect of bio-source type and appearance shape on the biomass heat capacity. Measurement. 2021 doi: 10.1016/J.MEASUREMENT.2021.110529. [DOI] [Google Scholar]
- Karimi M, Rodrigues AE, Silva JAC. Biomass as a source of adsorbents for CO2 capture. Adv Bioenergy Microfluid Appl. 2021 doi: 10.1016/B978-0-12-821601-9.00010-8. [DOI] [Google Scholar]
- Karimi M, Rodrigues AE, Silva JAC. Designing a simple volumetric apparatus for measuring gas adsorption equilibria and kinetics of sorption. Application and validation for CO2, CH4 and N2 adsorption in binder-free beads of 4A zeolite. Chem Eng J. 2021;425:130538. doi: 10.1016/J.CEJ.2021.130538. [DOI] [Google Scholar]
- Karimi M, Vaferi B, Hosseini SH, et al. Smart computing approach for design and scale-up of conical spouted beds with open-sided draft tubes. Particuology. 2021;55:179–190. doi: 10.1016/J.PARTIC.2020.09.003. [DOI] [Google Scholar]
- Karimi M, Khosravi M, Fathollahi R, et al. Determination of the heat capacity of cellulosic biosamples employing diverse machine learning approaches. Energy Sci Eng. 2022 doi: 10.1002/ESE3.1155. [DOI] [Google Scholar]
- Karimi M, Shirzad M, Silva JAC, Rodrigues AE. Biomass/biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: a review and prospects for future directions. J CO2 Util. 2022;57:101890. doi: 10.1016/J.JCOU.2022.101890. [DOI] [Google Scholar]
- Kearns D. Technology readiness and costs of CCS. Docklands: Global CCS Institute; 2021. [Google Scholar]
- Keith DW, Holmes G, St. Angelo D, Heidel K. A process for capturing CO2 from the atmosphere. Joule. 2018;2:1573–1594. doi: 10.1016/J.JOULE.2018.05.006. [DOI] [Google Scholar]
- Keller JU, Staudt R, Tomalla M. Volume-gravimetric measurements of binary gas adsorption equilibria. Ber Bunsenges Phys Chem. 1992;96:28–32. doi: 10.1002/BBPC.19920960105. [DOI] [Google Scholar]
- Keller JU, Robens E, von Hohenesche CDF. Thermogravimetric and sorption measurement techniques/instruments. Stud Surf Sci Catal. 2002;144:387–394. doi: 10.1016/S0167-2991(02)80159-8. [DOI] [Google Scholar]
- Keller JU, Staudt R (2005) Gas adsorption equilibria: experimental methods and adsorptive isotherms. In: Gas adsorption equilibria: experimental methods and adsorptive isotherms, pp 1–422. 10.1007/B102056/COVER
- Kent K (2009) Temperature swing adsorption system, US Patent 8353978B2. US Patent-US8353978B2
- Khajeh M, Ghaemi A. Exploiting response surface methodology for experimental modeling and optimization of CO2 adsorption onto NaOH-modified nanoclay montmorillonite. J Environ Chem Eng. 2020;8:103663. doi: 10.1016/J.JECE.2020.103663. [DOI] [Google Scholar]
- Khurana M, Farooq S. Integrated adsorbent process optimization for minimum cost of electricity including carbon capture by a VSA process. AIChE J. 2019;65:184–195. doi: 10.1002/AIC.16362. [DOI] [Google Scholar]
- Kohlheb N, Wluka M, Bezama A, et al. Environmental-economic assessment of the pressure swing adsorption biogas upgrading technology. Bioenergy Res. 2021;14:901–909. doi: 10.1007/S12155-020-10205-9/FIGURES/5. [DOI] [Google Scholar]
- Kumar R, Raut D, Ramamurty U, Rao CNR. Remarkable improvement in the mechanical properties and CO2 uptake of MOFs brought about by covalent linking to graphene. Angew Chem Int Ed Engl. 2016;55:7857–7861. doi: 10.1002/ANIE.201603320. [DOI] [PubMed] [Google Scholar]
- Kumar S, Srivastava R, Koh J. Utilization of zeolites as CO2 capturing agents: advances and future perspectives. J CO2 Util. 2020;41:101251. doi: 10.1016/J.JCOU.2020.101251. [DOI] [Google Scholar]
- Lachawiec AJ, DiRaimondo TR, Yang RT. A robust volumetric apparatus and method for measuring high pressure hydrogen storage properties of nanostructured materials. Rev Sci Instrum. 2008;79:063906. doi: 10.1063/1.2937820. [DOI] [PubMed] [Google Scholar]
- Lackner KS. The thermodynamics of direct air capture of carbon dioxide. Energy. 2013;50:38–46. doi: 10.1016/J.ENERGY.2012.09.012. [DOI] [Google Scholar]
- Lackner KS, Ziock H, Grimes P (1999) Carbon dioxide extraction from air: is it an option? In: Proceedings of the 24th annual technical conference on coal utilization & fuel systems, Clearwater. Accessed 15 Oct 2022. https://www.osti.gov/biblio/770509
- Lahtinen E, Precker RLM, Lahtinen M, et al. Selective laser sintering of metal–organic frameworks: production of highly porous filters by 3D printing onto a polymeric matrix. ChemPlusChem. 2019;84:222–225. doi: 10.1002/CPLU.201900081. [DOI] [PubMed] [Google Scholar]
- Lai JY, Ngu LH, Hashim SS. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh Gases Sci Technol. 2021;11:1076–1117. doi: 10.1002/GHG.2112. [DOI] [Google Scholar]
- Lawson S, Rezaei F. Effects of process parameters on CO2/H2 separation performance of 3D-printed MOF-74 monoliths. ACS Sustain Chem Eng. 2021;9:10902–10912. doi: 10.1021/acssuschemeng.1c03443. [DOI] [Google Scholar]
- Lawson S, Alwakwak A-A, Rownaghi AA, Rezaei F. Gel–print–grow: a new way of 3D printing metal-organic frameworks. ACS Appl Mater Interfaces. 2020;12:56108–56117. doi: 10.1021/acsami.0c18720. [DOI] [PubMed] [Google Scholar]
- Lawson S, Li X, Thakkar H, et al. Recent advances in 3D printing of structured materials for adsorption and catalysis applications. Chem Rev. 2021;121:6246–6291. doi: 10.1021/ACS.CHEMREV.1C00060/ASSET/IMAGES/LARGE/CR1C00060_0041.JPEG. [DOI] [PubMed] [Google Scholar]
- Lee SY, Park SJ. A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem. 2015;23:1–11. doi: 10.1016/J.JIEC.2014.09.001. [DOI] [Google Scholar]
- Lemaire P, Lee D, Zhao J, Parsons G. Reversible low-temperature metal node distortion during atomic layer deposition of Al2O3 and TiO2 on UiO-66-NH2 metal–organic framework crystal surfaces. ACS Appl Mater Interfaces. 2017;9:22042–22054. doi: 10.1021/acsami.7b05214. [DOI] [PubMed] [Google Scholar]
- Leonzio G, Mwabonje O, Fennell PS, Shah N. Environmental performance of different sorbents used for direct air capture. Sustain Prod Consum. 2022;32:101–111. doi: 10.1016/J.SPC.2022.04.004. [DOI] [Google Scholar]
- Leperi KT, Yancy-Caballero D, Snurr QR, You F. 110th anniversary: surrogate models based on artificial neural networks to simulate and optimize pressure swing adsorption cycles for CO2 capture. Ind Eng Chem Res. 2019;58:18241–18252. doi: 10.1021/acs.iecr.9b02383. [DOI] [Google Scholar]
- Leung DYC, Caramanna G, Maroto-Valer MM. An overview of current status of carbon dioxide capture and storage technologies. Renew Sustain Energy Rev. 2014;39:426–443. doi: 10.1016/J.RSER.2014.07.093. [DOI] [Google Scholar]
- Li H, Eddaoudi M, O’Keeffe M, Yaghi OM. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature. 1999;402:6759. doi: 10.1038/46248. [DOI] [Google Scholar]
- Li G, Xiao P, Xu D, Webley PA. Dual mode roll-up effect in multicomponent non-isothermal adsorption processes with multilayered bed packing. Chem Eng Sci. 2011;66:1825–1834. doi: 10.1016/J.CES.2011.01.023. [DOI] [Google Scholar]
- Li D, Tian Y, Li L, et al. Production of highly microporous carbons with large CO2 uptakes at atmospheric pressure by KOH activation of peanut shell char. J Porous Mater. 2015;22:1581–1588. doi: 10.1007/S10934-015-0041-7/TABLES/3. [DOI] [Google Scholar]
- Li W, Li Q, Zheng L, et al. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour Technol. 2015;194:276–282. doi: 10.1016/J.BIORTECH.2015.06.112. [DOI] [PubMed] [Google Scholar]
- Liao PQ, Chen H, Zhou DD, et al. Monodentate hydroxide as a super strong yet reversible active site for CO2 capture from high-humidity flue gas. Energy Environ Sci. 2015;8:1011–1016. doi: 10.1039/C4EE02717E. [DOI] [Google Scholar]
- Lin Y, Lin H, Wang H, et al. Enhanced selective CO2 adsorption on polyamine/MIL-101(Cr) composites. J Mater Chem A Mater. 2014;2:14658–14665. doi: 10.1039/C4TA01174K. [DOI] [Google Scholar]
- Lin JB, Nguyen TTT, Vaidhyanathan R, et al. A scalable metal–organic framework as a durable physisorbent for carbon dioxide capture. Science. 2021;374:1464–1469. doi: 10.1126/SCIENCE.ABI7281/SUPPL_FILE/SCIENCE.ABI7281_SM.PDF. [DOI] [PubMed] [Google Scholar]
- Liu Z, Grande CA, Li P, et al. Multi-bed vacuum pressure swing adsorption for carbon dioxide capture from flue gas. Sep Purif Technol. 2011;81:307–317. doi: 10.1016/J.SEPPUR.2011.07.037. [DOI] [Google Scholar]
- Liu S, Sun L, Xu F, et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ Sci. 2013;6:818–823. doi: 10.1039/C3EE23421E. [DOI] [Google Scholar]
- Liu W-J, Jiang H, Tian K, et al. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ Sci Technol. 2013;47:9397–9403. doi: 10.1021/es401286p. [DOI] [PubMed] [Google Scholar]
- Liu RS, Shi XD, Wang CT, et al. Advances in post-combustion CO2 capture by physical adsorption: from materials innovation to separation practice. ChemSusChem. 2021;14:1428–1471. doi: 10.1002/CSSC.202002677. [DOI] [PubMed] [Google Scholar]
- Liu X, Lim GJH, Wang Y, et al. Binder-free 3D printing of covalent organic framework (COF) monoliths for CO2 adsorption. Chem Eng J. 2021;403:126333. doi: 10.1016/J.CEJ.2020.126333. [DOI] [Google Scholar]
- Luberti M, Ahn H. Review of Polybed pressure swing adsorption for hydrogen purification. Int J Hydrogen Energy. 2022;47:10911–10933. doi: 10.1016/J.IJHYDENE.2022.01.147. [DOI] [Google Scholar]
- Lyu H, Li H, Hanikel N, et al. Covalent organic frameworks for carbon dioxide capture from air. J Am Chem Soc. 2022;144:12989–12995. doi: 10.1021/jacs.2c05382. [DOI] [PubMed] [Google Scholar]
- Madejski P, Chmiel K, Subramanian N, Kuś T. Methods and techniques for CO2 capture: review of potential solutions and applications in modern energy technologies. Energies. 2022;15(887 15):887. doi: 10.3390/EN15030887. [DOI] [Google Scholar]
- Manuel Vicent-Luna J, Jose Gutiérrez-Sevillano J, Hamad S, et al. Role of ionic liquid [EMIM]+[SCN]− in the adsorption and diffusion of gases in metal–organic frameworks. ACS Appl Mater Interfaces. 2018;10:29694–29704. doi: 10.1021/acsami.8b11842. [DOI] [PubMed] [Google Scholar]
- Mason J, McDonald MT, Bae T-H, et al. Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J Am Chem Soc. 2015;137:4787–4803. doi: 10.1021/jacs.5b00838. [DOI] [PubMed] [Google Scholar]
- Mendes DNDL, Gaspar A, Ferreira I, et al. 3D-printed hybrid zeolitic/carbonaceous electrically conductive adsorbent structures. Chem Eng Res Des. 2021;174:442–453. doi: 10.1016/J.CHERD.2021.08.020. [DOI] [Google Scholar]
- Miller DC, Litynski JT, Brickett LA, Morreale BD. Toward transformational carbon capture systems. AIChE J. 2016;62:2–10. doi: 10.1002/AIC.15066. [DOI] [Google Scholar]
- Minnick DL, Turnaoglu T, Rocha MA, Shiflett MB. Review Article: Gas and vapor sorption measurements using electronic beam balances. J Vac Sci Technol A Vac Surf Films. 2018;36:050801. doi: 10.1116/1.5044552. [DOI] [Google Scholar]
- Minx JC, Lamb WF, Callaghan MW, et al. Negative emissions—Part 1: research landscape and synthesis. Environ Res Lett. 2018;13:063001. doi: 10.1088/1748-9326/AABF9B. [DOI] [Google Scholar]
- Mitsuka Y, Ogiwara N, Mukoyoshi M, et al. Fabrication of integrated copper-based nanoparticles/amorphous metal–organic framework by a facile spray-drying method: highly enhanced CO2 hydrogenation activity for methanol synthesis. Angew Chem. 2021;133:22457–22462. doi: 10.1002/ANGE.202110585. [DOI] [PubMed] [Google Scholar]
- Moghadam PZ, Rogge SMJ, Li A, et al. Structure-mechanical stability relations of metal–organic frameworks via machine learning. Matter. 2019;1:219–234. doi: 10.1016/J.MATT.2019.03.002. [DOI] [Google Scholar]
- Moosavi M, Maik Jablonka K, Smit B. The role of machine learning in the understanding and design of materials. J Am Chem Soc. 2020;142:20273–20287. doi: 10.1021/jacs.0c09105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz R, Meier L, Diaz I, Jeison D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev Environ Sci Bio/technol. 2015;14(4):727–759. doi: 10.1007/S11157-015-9379-1. [DOI] [Google Scholar]
- Muralikrishna IV, Manickam V. Life cycle assessment. In: Muralikrishna IV, Manickam V, editors. Environmental management science and engineering for industry. 1. Elsevier; 2017. pp. 57–75. [Google Scholar]
- Na B-K, Lee H, Koo K-K, Keun Song H. Effect of rinse and recycle methods on the pressure swing adsorption process to recover CO2 from power plant flue gas using activated carbon. Ind Eng Chem Res. 2002;41:5498–5503. doi: 10.1021/ie0109509. [DOI] [Google Scholar]
- Nandi S, Collins S, Chakraborty D, et al. Ultralow parasitic energy for postcombustion CO2 capture realized in a nickel isonicotinate metal–organic framework with excellent moisture stability. J Am Chem Soc. 2017;139:1734–1737. doi: 10.1021/JACS.6B10455/SUPPL_FILE/JA6B10455_SI_002.CIF. [DOI] [PubMed] [Google Scholar]
- Naquash A, Qyyum MA, Haider J, et al. State-of-the-art assessment of cryogenic technologies for biogas upgrading: energy, economic, and environmental perspectives. Renew Sustain Energy Rev. 2022;154:111826. doi: 10.1016/J.RSER.2021.111826. [DOI] [Google Scholar]
- NASAClimate (2022a) https://climate.nasa.gov/vital-signs/global-temperature/, 2022a
- NASAClimate (2022b) https://climate.nasa.gov/vital-signs/carbon-dioxide/. Accessed 1 Nov 2022b
- Nasri NS, Hamza UD, Ismail SN, et al. Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J Clean Prod. 2014;71:148–157. doi: 10.1016/J.JCLEPRO.2013.11.053. [DOI] [Google Scholar]
- Nguyen HGT, Horn JC, Thommes M, et al. Experimental aspects of buoyancy correction in measuring reliable high-pressure excess adsorption isotherms using the gravimetric method. Meas Sci Technol. 2017;28:125802. doi: 10.1088/1361-6501/AA8F83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis GN, Kikkinides ES, Georgiadis MC. A model-based approach for the evaluation of new zeolite 13X-based adsorbents for the efficient post-combustion CO2 capture using P/VSA processes. Chem Eng Res Des. 2018;131:362–374. doi: 10.1016/J.CHERD.2017.06.016. [DOI] [Google Scholar]
- Nowrouzi M, Abyar H, Younesi H, Khaki E. Life cycle environmental and economic assessment of highly efficient carbon-based CO2 adsorbents: a comparative study. J CO2 Util. 2021;47:101491. doi: 10.1016/J.JCOU.2021.101491. [DOI] [Google Scholar]
- OGCI (2022) https://www.ogci.com/climate-investments/
- Ogungbenro AE, Quang DV, Al-Ali KA, et al. Synthesis and characterization of activated carbon from biomass date seeds for carbon dioxide adsorption. J Environ Chem Eng. 2020;8:104257. doi: 10.1016/J.JECE.2020.104257. [DOI] [Google Scholar]
- Olajire AA. Recent advances in the synthesis of covalent organic frameworks for CO2 capture. J CO2 Util. 2017;17:137–161. doi: 10.1016/J.JCOU.2016.12.003. [DOI] [Google Scholar]
- Osman AI, Hefny M, Abdel Maksoud MIA, et al. Recent advances in carbon capture storage and utilisation technologies: a review. Environ Chem Lett. 2020;19(2):797–849. doi: 10.1007/S10311-020-01133-3. [DOI] [Google Scholar]
- Osman AI, Chen L, Yang M, et al. Cost, environmental impact, and resilience of renewable energy under a changing climate: a review. Environ Chem Lett. 2022;1:1–24. doi: 10.1007/S10311-022-01532-8/TABLES/1. [DOI] [Google Scholar]
- OurWorldinData (2022) https://ourworldindata.org/Our World in Data
- Pachfule P, Banerjee R. Porous nitrogen rich cadmium-tetrazolate based metal organic framework (MOF) for H2 and CO2 uptake. Cryst Growth Des. 2011;11:5176–5181. doi: 10.1021/cg201054f. [DOI] [Google Scholar]
- Pai KN, Prasad V, Rajendran A. Experimentally validated machine learning frameworks for accelerated prediction of cyclic steady state and optimization of pressure swing adsorption processes. Sep Purif Technol. 2020;241:116651. doi: 10.1016/J.SEPPUR.2020.116651. [DOI] [Google Scholar]
- Palakkal AS, Pillai RS. Evaluating the performance of Cr-Soc-MOF super-adsorbents for CO2 capture from flue gas under humid condition through molecular simulation. Sep Purif Technol. 2022;295:121298. doi: 10.1016/J.SEPPUR.2022.121298. [DOI] [Google Scholar]
- Pan M, Omar HM, Rohani S. Application of nanosize zeolite molecular sieves for medical oxygen concentration. Nanomaterials. 2017;7(195 7):195. doi: 10.3390/NANO7080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascanu MM, Puig-Gamero M, Soreanu G, et al. Comparison of three Mexican biomasses valorization through combustion and gasification: environmental and economic analysis. Energy. 2019;189:116095. doi: 10.1016/J.ENERGY.2019.116095. [DOI] [Google Scholar]
- Paswan SK, Pradhan LK, Kumar P, et al. High-pressure adsorption measurements. A comparative study of the volumetric and gravimetric methods. Meas Sci Technol. 2004;15:848. doi: 10.1088/0957-0233/15/5/010. [DOI] [Google Scholar]
- Patra BR, Mukherjee A, Nanda S, Dalai AK. Biochar production, activation and adsorptive applications: a review. Environ Chem Lett. 2021;19(3):2237–2259. doi: 10.1007/S10311-020-01165-9. [DOI] [Google Scholar]
- Pereira A, Ferreira AFP, Rodrigues AE, et al. Additive manufacturing for adsorption-related applications—a review. J Adv Manuf Process. 2022;4:e10108. doi: 10.1002/AMP2.10108. [DOI] [Google Scholar]
- Permyakova A, Skrylnyk O, Courbon E, et al. Synthesis optimization, shaping, and heat reallocation evaluation of the hydrophilic metal–organic framework MIL-160(Al) ChemSusChem. 2017;10:1419–1426. doi: 10.1002/CSSC.201700164. [DOI] [PubMed] [Google Scholar]
- Petkovska M, Tondeur D, Grevillot G, et al. Temperature-swing gas separation with electrothermal desorption step. Sep Sci Technol. 2006;26:425–444. doi: 10.1080/01496399108050482. [DOI] [Google Scholar]
- Pettinari C, Tombesi A. Metal–organic frameworks for carbon dioxide capture. MRS Energy Sustain. 2020 doi: 10.1557/mre.2020.30. [DOI] [Google Scholar]
- Pietschak A, Kaiser M, Freund H. Tailored catalyst pellet specification for improved fixed-bed transport characteristics: a shortcut method for the model-based reactor design. Chem Eng Res Des. 2018;137:60–74. doi: 10.1016/J.CHERD.2018.06.043. [DOI] [Google Scholar]
- Plaza MG, González AS, Pevida C, et al. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl Energy. 2012;99:272–279. doi: 10.1016/J.APENERGY.2012.05.028. [DOI] [Google Scholar]
- Pohanka M. Three-dimensional printing in analytical chemistry: principles and applications. Anal Lett. 2016;49:2865–2882. doi: 10.1080/00032719.2016.1166370. [DOI] [Google Scholar]
- Presser V, McDonough J, Yeon SH, Gogotsi Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ Sci. 2011;4:3059–3066. doi: 10.1039/C1EE01176F. [DOI] [Google Scholar]
- Pu S, Wang J, Li L, et al. Performance comparison of metal–organic framework extrudates and commercial zeolite for ethylene/ethane separation. Ind Eng Chem Res. 2018;57:1645–1654. doi: 10.1021/acs.iecr.7b04391. [DOI] [Google Scholar]
- Pullumbi P, Brandani F, Brandani S. Gas separation by adsorption: technological drivers and opportunities for improvement. Curr Opin Chem Eng. 2019;24:131–142. doi: 10.1016/J.COCHE.2019.04.008. [DOI] [Google Scholar]
- Rabbani MG, Sekizkardes AK, Kahveci Z, et al. A 2D mesoporous imine-linked covalent organic framework for high pressure gas storage applications. Chem A Eur J. 2013;19:3324–3328. doi: 10.1002/CHEM.201203753. [DOI] [PubMed] [Google Scholar]
- Rashidi NA, Yusup S. Co-valorization of delayed petroleum coke—palm kernel shell for activated carbon production. J Hazard Mater. 2021;403:123876. doi: 10.1016/J.JHAZMAT.2020.123876. [DOI] [PubMed] [Google Scholar]
- Regufe MJ, Ferreira AFP, Loureiro JM, et al. Electrical conductive 3D-printed monolith adsorbent for CO2 capture. Microporous Mesoporous Mater. 2019;278:403–413. doi: 10.1016/J.MICROMESO.2019.01.009. [DOI] [Google Scholar]
- Regufe MJ, Pereira A, Ferreira AFP, et al. Current developments of carbon capture storage and/or utilization-looking for net-zero emissions defined in the Paris Agreement. Energies. 2021;14:2406–2406. doi: 10.3390/EN14092406. [DOI] [Google Scholar]
- Rezaei F, Webley P. Optimum structured adsorbents for gas separation processes. Chem Eng Sci. 2009;64:5182–5191. doi: 10.1016/J.CES.2009.08.029. [DOI] [Google Scholar]
- Ribeiro RPPL, Grande CA, Rodrigues AE. Electric swing adsorption for gas separation and purification: a review. Sep Sci Technol. 2014;49:1985–2002. doi: 10.1080/01496395.2014.915854. [DOI] [Google Scholar]
- Riboldi L. Assessment of pressure swing adsorption as CO2 capture technology in coal-fired power plants. NTNU; 2016. [Google Scholar]
- Roque-Malherbe RMA. Adsorption and diffusion in nanoporous materials. 2. CRC Press; 2018. [Google Scholar]
- Rosen AS, Mian MR, Islamoglu T, et al. Tuning the redox activity of metal–organic frameworks for enhanced, selective O2 binding: design rules and ambient temperature O2 chemisorption in a cobalt-triazolate framework. J Am Chem Soc. 2020;142:4317–4328. doi: 10.1021/JACS.9B12401/ASSET/IMAGES/LARGE/JA9B12401_0009.JPEG. [DOI] [PubMed] [Google Scholar]
- Rubio-Martinez M, Avci-Camur C, Thornton AW, et al. New synthetic routes towards MOF production at scale. Chem Soc Rev. 2017;46:3453–3480. doi: 10.1039/C7CS00109F. [DOI] [PubMed] [Google Scholar]
- Ruthven DM. Principles of adsorption and adsorption processes. 1. New York: Wiley; 1984. [Google Scholar]
- Ruthven DM. Report of PSA discussion. Stud Surf Sci Catal. 1993;80:787–793. doi: 10.1016/S0167-2991(08)63590-9. [DOI] [Google Scholar]
- Ruthven DM. CO2 capture: value functions, separative work and process economics. Chem Eng Sci. 2014;114:128–133. doi: 10.1016/J.CES.2014.04.020. [DOI] [Google Scholar]
- Sangchoom W, Mokaya R. Valorization of lignin waste: carbons from hydrothermal carbonization of renewable lignin as superior sorbents for CO2 and hydrogen storage. ACS Sustain Chem Eng. 2015;3:1658–1667. doi: 10.1021/acssuschemeng.5b00351. [DOI] [Google Scholar]
- Sani R, Dey TK, Sarkar M, et al. A study of contemporary progress relating to COF materials for CO2 capture and fixation reactions. Mater Adv. 2022;3:5575–5597. doi: 10.1039/D2MA00143H. [DOI] [Google Scholar]
- Saosee P, Sajjakulnukit B, Gheewala SH. Life cycle assessment of wood pellet production in Thailand. Sustainability. 2020;12:6996. doi: 10.3390/SU12176996. [DOI] [Google Scholar]
- Sargazi G, Afzali D, Mostafavi A, Ebrahimipour SY. Ultrasound-assisted facile synthesis of a new tantalum(V) metal–organic framework nanostructure: Design, characterization, systematic study, and CO2 adsorption performance. J Solid State Chem. 2017;250:32–48. doi: 10.1016/J.JSSC.2017.03.014. [DOI] [Google Scholar]
- Scarlat N, Dallemand JF, Fahl F. Biogas: developments and perspectives in Europe. Renew Energy. 2018;129:457–472. doi: 10.1016/J.RENENE.2018.03.006. [DOI] [Google Scholar]
- Schmidt T, Beuse M, Zhang X, et al. Additional emissions and cost from storing electricity in stationary battery systems. Environ Sci Technol. 2019;53:3379–3390. doi: 10.1021/acs.est.8b05313. [DOI] [PubMed] [Google Scholar]
- Serna-Guerrero R, Da’na E, Sayari A. New Insights into the Interactions of CO2 with amine-functionalized silica. Ind Eng Chem Res. 2008;47:9406–9412. doi: 10.1021/ie801186g. [DOI] [Google Scholar]
- Sevilla M, Fuertes AB. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ Sci. 2011;4:1765–1771. doi: 10.1039/C0EE00784F. [DOI] [Google Scholar]
- Sevilla M, Falco C, Titirici MM, Fuertes AB. High-performance CO2 sorbents from algae. RSC Adv. 2012;2:12792–12797. doi: 10.1039/C2RA22552B. [DOI] [Google Scholar]
- Sha Y, Lou J, Bai S, et al. Facile preparation of nitrogen-doped porous carbon from waste tobacco by a simple pre-treatment process and their application in electrochemical capacitor and CO2 capture. Mater Res Bull. 2015;64:327–332. doi: 10.1016/J.MATERRESBULL.2015.01.015. [DOI] [Google Scholar]
- Shade D, Bout WSB, Sholl SD, Walton SK. Opening the toolbox: 18 experimental techniques for measurement of mixed gas adsorption. Ind Eng Chem Res. 2022;61:2367–2391. doi: 10.1021/acs.iecr.1c03756. [DOI] [Google Scholar]
- Shamsudin IK, Idris I, Abdullah A, et al. Development of microporous Zr-MOF UiO-66 by sol-gel synthesis for CO2 capture from synthetic gas containing CO2 and H2. AIP Conf Proc. 2019;2124:020057. doi: 10.1063/1.5117117. [DOI] [Google Scholar]
- Sharma A, Jindal J, Mittal A, et al. Carbon materials as CO2 adsorbents: a review. Environ Chem Lett. 2021;19(2):875–910. doi: 10.1007/S10311-020-01153-Z. [DOI] [Google Scholar]
- Shekhah O, Belmabkhout Y, Chen Z, et al. Made-to-order metal–organic frameworks for trace carbon dioxide removal and air capture. Nat Commun. 2014;5(1):1–7. doi: 10.1038/ncomms5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Cortright RD, Chen Y, Dumesic JA. Microcalorimetric and Infrared Spectroscopic Studies of.gamma.-Al2O3 Modified by Basic Metal Oxides. J Phys Chem. 2002;98:8067–8073. doi: 10.1021/j100084a025. [DOI] [Google Scholar]
- Shirzad M, Karimi M, Abolghasemi H. Polymer inclusion membranes with dinonylnaphthalene sulfonic acid as ion carrier for Co(II) transport from model solutions. Desalin Water Treat. 2019;144:185–200. doi: 10.5004/dwt.2019.23575. [DOI] [Google Scholar]
- Shirzad M, Kazemi Shariat Panahi H, Dashti BB, et al. A comprehensive review on electricity generation and GHG emission reduction potentials through anaerobic digestion of agricultural and livestock/slaughterhouse wastes in Iran. Renew Sustain Energy Rev. 2019;111:571–594. doi: 10.1016/J.RSER.2019.05.011. [DOI] [Google Scholar]
- Sieverts A. Information on the occlusion and diffusion of gases through metals. Z Phys Chem. 1907;60:129–201. doi: 10.1515/zpch-1907-6009. [DOI] [Google Scholar]
- Silalertruksa T, Gheewala SH. Competitive use of sugarcane for food, fuel, and biochemical through the environmental and economic factors. Int J Life Cycle Assess. 2020;25:1343–1355. doi: 10.1007/S11367-019-01664-0/TABLES/3. [DOI] [Google Scholar]
- Siqueira RM, Freitas GR, Peixoto HR, et al. Carbon dioxide capture by pressure swing adsorption. Energy Procedia. 2017;114:2182–2192. doi: 10.1016/J.EGYPRO.2017.03.1355. [DOI] [Google Scholar]
- Sircar S. Basic research needs for design of adsorptive gas separation processes. Ind Eng Chem Res. 2006;45:5435–5448. doi: 10.1021/ie051056a. [DOI] [Google Scholar]
- Siriwardane R, Shen M-S, Fisher EP, Losch J. Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels. 2005;19:1153–1159. doi: 10.1021/ef040059h. [DOI] [Google Scholar]
- Skarstrom CW (1960) Method and apparatus for fractionating gaseous mixtures by adsorption. US Patent 2944627A
- Smith P, Davis SJ, Creutzig F, et al. Biophysical and economic limits to negative CO2 emissions. Nat Clim Change. 2015;6(1):42–50. doi: 10.1038/nclimate2870. [DOI] [Google Scholar]
- Song J, Shen W, Wang J, Fan W. Superior carbon-based CO2 adsorbents prepared from poplar anthers. Carbon N Y. 2014;69:255–263. doi: 10.1016/J.CARBON.2013.12.024. [DOI] [Google Scholar]
- Songolzadeh M, Ravanchi MT, Soleimani M. Carbon dioxide capture and storage: a general review on adsorbents. Int J Chem Mol Eng. 2012;6:906–913. doi: 10.5281/ZENODO.1076266. [DOI] [Google Scholar]
- Spitoni M, Pierantozzi M, Comodi G, et al. Theoretical evaluation and optimization of a cryogenic technology for carbon dioxide separation and methane liquefaction from biogas. J Nat Gas Sci Eng. 2019;62:132–143. doi: 10.1016/J.JNGSE.2018.12.007. [DOI] [Google Scholar]
- Sreńscek-Nazzal J, Kiełbasa K. Advances in modification of commercial activated carbon for enhancement of CO2 capture. Appl Surf Sci. 2019;494:137–151. doi: 10.1016/J.APSUSC.2019.07.108. [DOI] [Google Scholar]
- Sreńscek-Nazzal J, Urszula Narkiewicz U, Antoni W, Morawski A, et al. Modification of commercial activated carbons for CO2 adsorption. Acta Phys Polonica Ser A. 2016;129:394–401. doi: 10.12693/APhysPolA.129.394. [DOI] [Google Scholar]
- Stark T (1963) Gas separation by adsorption process. US Patent 3252268A
- Stassen I, Styles M, van Assche T, et al. Electrochemical film deposition of the zirconium metal–organic framework UiO-66 and application in a miniaturized sorbent trap. Chem Mater. 2015;27:1801–1807. doi: 10.1021/cm504806p. [DOI] [Google Scholar]
- Steldinger H, Esposito A, Brunnengräber K, et al. 3D printing: activated carbon in the third dimension—3D printing of a tuned porous carbon (Adv. Sci. 19/2019) Adv Sci. 2019;6:1970114. doi: 10.1002/ADVS.201970114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian Balashankar V, Rajendran A. Process optimization-based screening of zeolites for post-combustion CO2 capture by vacuum swing adsorption. ACS Sustain Chem Eng. 2019;7:17747–17755. doi: 10.1021/acssuschemeng.9b04124. [DOI] [Google Scholar]
- Subraveti SG, Li Z, Prasad V, Rajendran A. Machine learning-based multiobjective optimization of pressure swing adsorption. Ind Eng Chem Res. 2019;58:20412–20422. doi: 10.1021/acs.iecr.9b04173. [DOI] [Google Scholar]
- Suyetin M. Rht-MOFs with triptycene-modified linkers for balanced gravimetric/volumetric hydrogen storage: a molecular simulation study. Int J Hydrogen Energy. 2017;42:3114–3121. doi: 10.1016/J.IJHYDENE.2017.01.062. [DOI] [Google Scholar]
- SvanteInc (2021) https://svanteinc.com/carbon-capture-technology/, 2021
- SvanteInc (2022) https://svanteinc.com/2021/12/16/mof-sorbent-on-a-roll-a-scalable-solution-for-gigaton-scale-carbon-capture/
- Tagliabue M, Rizzo C, Onorati NB, et al. Regenerability of zeolites as adsorbents for natural gas sweetening: a case-study. Fuel. 2012;93:238–244. doi: 10.1016/J.FUEL.2011.08.051. [DOI] [Google Scholar]
- Takeda Y, Okumura S, Tone S, et al. Cyclizative atmospheric CO2 fixation by unsaturated amines with t-BuOI leading to cyclic carbamates. Org Lett. 2012;14:4874–4877. doi: 10.1021/ol302201q. [DOI] [PubMed] [Google Scholar]
- TakhtRavanchi M, Sahebdelfar S. Carbon dioxide capture and utilization in petrochemical industry: potentials and challenges. Appl Petrochem Res. 2014;4(1):63–77. doi: 10.1007/S13203-014-0050-5. [DOI] [Google Scholar]
- TakhtRavanchi M, Sahebdelfar S. Catalytic conversions of CO2 to help mitigate climate change: recent process developments. Process Saf Environ Prot. 2021;145:172–194. doi: 10.1016/J.PSEP.2020.08.003. [DOI] [Google Scholar]
- TakhtRavanchi M, Sahebdelfar S, Zangeneh FT. Carbon dioxide sequestration in petrochemical industries with the aim of reduction in greenhouse gas emissions. Front Chem Eng China. 2011;5:173–178. doi: 10.1007/S11705-010-0562-1/METRICS. [DOI] [Google Scholar]
- Tang Y, You F. Multicriteria environmental and economic analysis of municipal solid waste incineration power plant with carbon capture and separation from the life-cycle perspective. ACS Sustain Chem Eng. 2017;6:937–956. doi: 10.1021/acssuschemeng.7b03283. [DOI] [Google Scholar]
- Tao Y, Xie X, Lv W, et al. Towards ultrahigh volumetric capacitance: graphene derived highly dense but porous carbons for supercapacitors. Sci Rep. 2013;3(1):1–8. doi: 10.1038/srep02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlouw T, Bauer C, Rosa L, Mazzotti M. Life cycle assessment of carbon dioxide removal technologies: a critical review. Energy Environ Sci. 2021;14:1701–1721. doi: 10.1039/D0EE03757E. [DOI] [Google Scholar]
- Terlouw T, Treyer K, Bauer C, Mazzotti M. Life cycle assessment of direct air carbon capture and storage with low-carbon energy sources. Environ Sci Technol. 2021;55:11397–11411. doi: 10.1021/acs.est.1c03263. [DOI] [PubMed] [Google Scholar]
- Thomas D, Benson S. Carbon dioxide capture for storage in deep geologic formations—results from the CO2 capture project. 1. Elsevier; 2005. [Google Scholar]
- Tribe MA, Alpine RLW. Scale economies and the “0.6 rule”. Eng Costs Prod Econ. 1986;10:271–278. doi: 10.1016/0167-188X(86)90053-4. [DOI] [Google Scholar]
- UNFCCC (2022) Adoption of the Paris Agreement. Proposal by the President. https://unfccc.int/documents/9064
- United Nations (2022) https://www.un.org/en/climatechange/what-is-climate-change. https://www.un.org/en/climatechange/what-is-climate-change, 2022. Accessed 1 Nov 2022
- Usman M, Helal A, Abdelnaby MM, et al. Trends and prospects in UiO-66 metal–organic framework for CO2 capture, separation, and conversion. Chem Rec. 2021;21:1771–1791. doi: 10.1002/TCR.202100030. [DOI] [PubMed] [Google Scholar]
- Vaitsis C, Kanellou E, Pandis PK, et al. Sonochemical synthesis of zinc adipate metal–organic framework (MOF) for the electrochemical reduction of CO2: MOF and circular economy potential. Sustain Chem Pharm. 2022;29:100786. doi: 10.1016/J.SCP.2022.100786. [DOI] [Google Scholar]
- Vakili R, Xu S, Al-Janabi N, et al. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): optimization and gas adsorption. Microporous Mesoporous Mater. 2018;260:45–53. doi: 10.1016/J.MICROMESO.2017.10.028. [DOI] [Google Scholar]
- Vivo-Vilches JF, Pérez-Cadenas AF, Maldonado-Hódar FJ, et al. Resorcinol–formaldehyde carbon xerogel as selective adsorbent of carbon dioxide present on biogas. Adsorption. 2018;24:169–177. doi: 10.1007/S10450-018-9933-6/TABLES/4. [DOI] [Google Scholar]
- Vo ND, Oh DH, Kang JH, et al. Dynamic-model-based artificial neural network for H2 recovery and CO2 capture from hydrogen tail gas. Appl Energy. 2020;273:115263. doi: 10.1016/J.APENERGY.2020.115263. [DOI] [Google Scholar]
- Wang Q, Luo J, Zhong Z, Borgna A. CO2 capture by solid adsorbents and their applications: current status and new trends. Energy Environ Sci. 2010;4:42–55. doi: 10.1039/C0EE00064G. [DOI] [Google Scholar]
- Wang J, Heerwig A, Lohe MR, et al. Fungi-based porous carbons for CO2 adsorption and separation. J Mater Chem. 2012;22:13911–13913. doi: 10.1039/C2JM32139D. [DOI] [Google Scholar]
- Wang L, Deng H, Furukawa H, et al. Synthesis and Characterization of metal–organic framework-74 containing 2, 4, 6, 8, and 10 different metals. Inorg Chem. 2014;53:5881–5883. doi: 10.1021/ic500434a. [DOI] [PubMed] [Google Scholar]
- Wang TH, Xiao DC, Huang CH, et al. CO2 uptake performance and life cycle assessment of CaO-based sorbents prepared from waste oyster shells blended with PMMA nanosphere scaffolds. J Hazard Mater. 2014;270:92–101. doi: 10.1016/J.JHAZMAT.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Wang B, Xie LH, Wang X, et al. Applications of metal–organic frameworks for green energy and environment: new advances in adsorptive gas separation, storage and removal. Green Energy Environ. 2018;3:191–228. doi: 10.1016/J.GEE.2018.03.001. [DOI] [Google Scholar]
- Wang D, Streater D, Peng Y, Huang J. 2D covalent organic frameworks with an incorporated manganese complex for light driven carbon dioxide reduction. ChemPhotoChem. 2021;5:1119–1123. doi: 10.1002/CPTC.202100123. [DOI] [Google Scholar]
- Wang Q, Yu F, Zhang M, et al. CO2 dual roles in food scraps-derived biochar activation to enhance lead adsorption capacity. Sci Total Environ. 2021;784:147218. doi: 10.1016/J.SCITOTENV.2021.147218. [DOI] [PubMed] [Google Scholar]
- Wawrzyńczak D, Panowski M, Majchrzak-Kucęba I. Possibilities of CO2 purification coming from oxy-combustion for enhanced oil recovery and storage purposes by adsorption method on activated carbon. Energy. 2019;180:787–796. doi: 10.1016/J.ENERGY.2019.05.068. [DOI] [Google Scholar]
- WBA (2022) Global bioenergy statistics, World bioenergy association (WBA), https://www.worldbioenergy.org/uploads/211214%20WBA%20GBS%202021.pdf
- Wei H, Chai S, Hu N, et al. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem Commun. 2015;51:12178–12181. doi: 10.1039/C5CC04680G. [DOI] [PubMed] [Google Scholar]
- Wilcox J, Haghpanah R, Rupp EC, et al. Advancing adsorption and membrane separation processes for the gigaton carbon capture challenge. Annu Rev Chem Biomol Eng. 2014;5:479–505. doi: 10.1146/ANNUREV-CHEMBIOENG-060713-040100. [DOI] [PubMed] [Google Scholar]
- Wilmer CE, Leaf M, Lee CY, et al. Large-scale screening of hypothetical metal–organic frameworks. Nat Chem. 2011;4(2):83–89. doi: 10.1038/nchem.1192. [DOI] [PubMed] [Google Scholar]
- WolfCarbon https://wolfcarbonsolutions.com. In: 2022
- Wu F, Zhou Z, Temizel-Sekeryan S, et al. Assessing the environmental impact and payback of carbon nanotube supported CO2 capture technologies using LCA methodology. J Clean Prod. 2020;270:122465. doi: 10.1016/J.JCLEPRO.2020.122465. [DOI] [Google Scholar]
- Xia W, Lau SK, Yong WF. Comparative life cycle assessment on zeolitic imidazolate framework-8 (ZIF-8) production for CO2 capture. J Clean Prod. 2022;370:133354. doi: 10.1016/J.JCLEPRO.2022.133354. [DOI] [Google Scholar]
- Xiang Z, Hu Z, Cao D, et al. Metal–organic frameworks with incorporated carbon nanotubes: improving carbon dioxide and methane storage capacities by lithium doping. Angew Chem Int Ed. 2011;50:491–494. doi: 10.1002/ANIE.201004537. [DOI] [PubMed] [Google Scholar]
- Xie LH, Suh MP. High CO2-capture ability of a porous organic polymer bifunctionalized with carboxy and triazole groups. Chem A Eur J. 2013;19:11590–11597. doi: 10.1002/CHEM.201301822. [DOI] [PubMed] [Google Scholar]
- Xu G, Li L, Yang Y, et al. A novel CO2 cryogenic liquefaction and separation system. Energy. 2012;42:522–529. doi: 10.1016/J.ENERGY.2012.02.048. [DOI] [Google Scholar]
- Xu L, Khalifeh A, Khandakar A, Vaferi B. Numerical investigating the effect of Al2O3–water nanofluids on the thermal efficiency of flat plate solar collectors. Energy Rep. 2022;8:6530–6542. doi: 10.1016/J.EGYR.2022.05.012. [DOI] [Google Scholar]
- Xu J, Weist EL (2000) Six bed pressure swing adsorption process with four steps of pressure equalization. US Patent US6454838B1
- Yan Y, Borhani TN, Subraveti SG, et al. Harnessing the power of machine learning for carbon capture, utilisation, and storage (CCUS)—a state-of-the-art review. Energy Environ Sci. 2021;14:6122–6157. doi: 10.1039/D1EE02395K. [DOI] [Google Scholar]
- Yang H, Xu Z, Fan M, et al. Progress in carbon dioxide separation and capture: a review. J Environ Sci. 2008;20:14–27. doi: 10.1016/S1001-0742(08)60002-9. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Jung H, Kim T, Park CR. Recent advances in hydrogen storage technologies based on nanoporous carbon materials. Progress Nat Sci Mater Int. 2012;22:631–638. doi: 10.1016/J.PNSC.2012.11.006. [DOI] [Google Scholar]
- Yang Y, Sitprasert C, Rufford TE, et al. An experimental and simulation study of binary adsorption in metal–organic frameworks. Sep Purif Technol. 2015;146:136–142. doi: 10.1016/J.SEPPUR.2015.03.041. [DOI] [Google Scholar]
- Yang S, Wei Y, Chen Z, et al. Effects of multicomponent adsorption on enhanced shale reservoir recovery by CO2 injection coupled with reservoir geomechanics. Soc Pet Eng SPE Low Perm Symp. 2016 doi: 10.2118/180208-MS. [DOI] [Google Scholar]
- Yang Z, Guo W, Mahurin SM, et al. Surpassing robeson upper limit for CO2/N2 separation with fluorinated carbon molecular sieve membranes. Chem. 2020;6:631–645. doi: 10.1016/J.CHEMPR.2019.12.006. [DOI] [Google Scholar]
- Yazaydin AÖ, Snurr RQ, Park TH, et al. Screening of metal–organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J Am Chem Soc. 2009;131:18198–18199. doi: 10.1021/JA9057234/SUPPL_FILE/JA9057234_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- Yazaydın AÖ, Snurr RQ, Park T-H, et al. Screening of metal−organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J Am Chem Soc. 2009;131:18198–18199. doi: 10.1021/ja9057234. [DOI] [PubMed] [Google Scholar]
- Yong Z, Mata V, Rodrigues AE. Adsorption of carbon dioxide at high temperature—a review. Sep Purif Technol. 2002;26:195–205. doi: 10.1016/S1383-5866(01)00165-4. [DOI] [Google Scholar]
- Younas M, Rezakazemi M, Daud M, et al. Recent progress and remaining challenges in post-combustion CO2 capture using metal–organic frameworks (MOFs) Prog Energy Combust Sci. 2020;80:100849. doi: 10.1016/J.PECS.2020.100849. [DOI] [Google Scholar]
- Yu CH, Huang CH, Tan CS. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual Res. 2012;12:745–769. doi: 10.4209/AAQR.2012.05.0132. [DOI] [Google Scholar]
- Yu J, Xie LH, Li JR, et al. CO2 capture and separations using MOFs: computational and experimental studies. Chem Rev. 2017;117:9674–9754. doi: 10.1021/ACS.CHEMREV.6B00626/ASSET/IMAGES/LARGE/CR-2016-006263_0039.JPEG. [DOI] [PubMed] [Google Scholar]
- Yulia F, Nasruddin ZA, Ruliandini R. Metal–organic framework based chromium terephthalate (MIL-101 Cr) growth for carbon dioxide capture: a review. J Adv Res Fluid Mech Thermal Sci. 2019;57:158–174. [Google Scholar]
- Zeman F. Energy and material balance of CO2 capture from ambient air. Environ Sci Technol. 2007;41:7558–7563. doi: 10.1021/es070874m. [DOI] [PubMed] [Google Scholar]
- Zeng W, Bai H. Swelling-agent-free synthesis of rice husk derived silica materials with large mesopores for efficient CO2 capture. Chem Eng J. 2014;251:1–9. doi: 10.1016/J.CEJ.2014.04.041. [DOI] [Google Scholar]
- Zhang C, Liu Y, Li B, et al. Triptycene-based microporous polymers: synthesis and their gas storage properties. ACS Macro Lett. 2011;1:190–193. doi: 10.1021/mz200076c. [DOI] [PubMed] [Google Scholar]
- Zhang X-X, Xiao P, Zhan C-H, et al. Separation of methane/ethylene gas mixtures using Wet ZIF-8. Ind Eng Chem Res. 2015;54:7890–7898. doi: 10.1021/acs.iecr.5b00941. [DOI] [Google Scholar]
- Zhang Y, Zhang P, Yu W, et al. Facile and controllable preparation of ultramicroporous biomass-derived carbons and application on selective adsorption of gas-mixtures. Ind Eng Chem Res. 2018;57:14191–14201. doi: 10.1021/acs.iecr.8b02139. [DOI] [Google Scholar]
- Zhang YH, Bai J, Chen Y, et al. A Zn(II)-based pillar-layered metal–organic framework: synthesis, structure, and CO2 selective adsorption. Polyhedron. 2019;158:283–289. doi: 10.1016/J.POLY.2018.10.067. [DOI] [Google Scholar]
- Zhang H, Wang P, Zhang H, et al. Structured zeolite monoliths with ultrathin framework for fast CO2 adsorption enabled by 3D printing. Ind Eng Chem Res. 2020;59:8223–8229. doi: 10.1021/acs.iecr.9b07060. [DOI] [Google Scholar]
- Zhao Y, Yao KX, Teng B, et al. A perfluorinated covalent triazine-based framework for highly selective and water–tolerant CO2 capture. Energy Environ Sci. 2013;6:3684–3692. doi: 10.1039/C3EE42548G. [DOI] [Google Scholar]
- Zhao R, Zhao L, Wang S, et al. Solar-assisted pressure-temperature swing adsorption for CO2 capture: effect of adsorbent materials. Sol Energy Mater Sol Cells. 2018;185:494–504. doi: 10.1016/J.SOLMAT.2018.06.004. [DOI] [Google Scholar]
- Zhou Z, Davoudi E, Vaferi B. Monitoring the effect of surface functionalization on the CO2 capture by graphene oxide/methyl diethanolamine nanofluids. J Environ Chem Eng. 2021;9:106202. doi: 10.1016/J.JECE.2021.106202. [DOI] [Google Scholar]
- Zhu C, Han TYJ, Duoss EB, et al. Highly compressible 3D periodic graphene aerogel microlattices. Nat Commun. 2015;6(1):1–8. doi: 10.1038/ncomms7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann AW, Langhorst T, Moni S, et al. Life-cycle and techno-economic assessment of early-stage carbon capture and utilization technologies—a discussion of current challenges and best practices. Front Clim. 2022;4:44. doi: 10.3389/FCLIM.2022.841907/BIBTEX. [DOI] [Google Scholar]
- Zubbri NA, Mohamed AR, Kamiuchi N, Mohammadi M. Enhancement of CO2 adsorption on biochar sorbent modified by metal incorporation. Environ Sci Pollut Res Int. 2020;27:11809–11829. doi: 10.1007/S11356-020-07734-3. [DOI] [PubMed] [Google Scholar]
- Zunita M, Natola OW, David M, Lugito G. Integrated metal organic framework/ionic liquid-based composite membrane for CO2 separation. Chem Eng J Adv. 2022;11:100320. doi: 10.1016/J.CEJA.2022.100320. [DOI] [Google Scholar]