Abstract
Background and aim:
Angiopoietin-like 3 (ANGPTL3) and 4 (ANGPTL4) are regulators of triglyceride storage and utilization. Bariatric surgery (BS) leads to profound changes in adipose tissue composition and energy metabolism. We evaluated the impact of BS on plasma levels of ANGPTL3 and ANGPTL4.
Methods and results:
Twenty-seven subjects affected by morbid obesity with or without type 2 diabetes (T2D) underwent Roux-en-Y gastric bypass (RYGB) and 18 patients with advanced T2D received Biliopancreatic Diversion (BPD). Fasting ANGPTL proteins levels, insulin sensitivity (evaluated by euglycemic hyperinsulinemic clamp), total bile acids (TBA) and free fatty acids (FFA) were measured at baseline and 1 year after surgery.
Both surgical procedures resulted in the loss of fat mass, improved glucose control, and a ~2-fold increase of insulin sensitivity. ANGPTL4 levels decreased significantly with both RYGB (26.6 ± 0.6 to 24.4 ± 0.3 ng/mL, p = 0.001) and BPD (27.9 ± 1.5 to 24.0 ± 0.5 ng/mL, p = 0.003). In contrast, ANGPTL3 concentrations did not change after RYGB but rose following BPD (225 ± 20 to 300 ± 15 ng/mL, p = 0.003). By multiple regression analysis, changes after BS in ANGPTL4 were independently associated with changes in blood glucose, (p = 0.0169) whereas changes in ANGPTL3 were associated with variations in FFA (p = 0.008) and insulin sensitivity (p = 0.043).
Conclusion:
Circulating ANGPTL4 is reduced by BS, probably due to the loss of fat mass and improved insulin sensitivity. Conversely, ANGPTL3 levels increased after BPD, but not after RYGB, presumably because of the metabolic changes induced by the malabsorptive effect of BPD.
Keywords: ANGPTL3, ANGPTL4, Bariatric surgery, Type 2 diabetes mellitus, Insulin sensitivity, Lipid metabolism
1. Introduction
Angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) are crucial regulators in the transport of triglyceride-rich lipoproteins (TRLs) [1]. ANGPTL3 is a secreted glycoprotein expressed almost exclusively by the liver, while ANGPTL4 is produced mainly by the liver and the adipose tissue [2]. Both angiopoietins act as inhibitors of lipoprotein lipase (LPL) [3,4]. More recently, it has been demonstrated that the inhibitory activity of ANGPTL3 and 4 is also modulated by another protein belonging to the angiopoietin-like family, angiopoietin-like protein 8 (ANGPTL8) [5]. ANGPTL8 is secreted in response to feeding. It has been reported that it is able to reduce lipogenesis in human hepatocytes exposed to lipotoxic conditions [6]. More importantly, it is able to form complexes with both ANGPTL3 (in a 3:1 M ratio) and ANGPTL4 (in 1:1 M ratio). The ANGPTL3/8 complex is 100-times more potent in LPL inhibition than ANGPTL3 alone, while the ANGPTL4/8 complex is 100-times less potent in inhibiting LPL than ANGPTL4 alone, thus establishing a possible hierarchical mechanism in regulating LPL-activity in different tissues [7].
LPL plays a fundamental role in the catabolism of circulating TRLs such as chylomicrons and very low-density lipoproteins (VLDL), by catalyzing the hydrolysis of their triglyceride (TG) content into free fatty acids (FFA). Consistently, the overexpression of ANGPTL3 and 4 in mice caused hypertriglyceridemia [8], and the genetically-determined complete deficiency of ANGPTL3 in humans is associated with increased LPL activity, low fasting concentration of plasma TG and increased removal of TRLs during the postprandial phase [9,10].
The function of ANGPTL proteins (ANGPTLs) probably goes beyond their effect in regulating TRL metabolism, as they may be involved in the adipose tissue physiology mainly by influencing the trafficking of FFA [2,11]. Available evidence exploring the action of ANGPTLs in adipose tissue is scarce and most published studies are based on murine models. Moreover, contradictory evidence has been reported on circulating levels of ANGPTL3 and 4 in subjects with obesity [12-14]. Nevertheless, in a condition where the body distribution of adipose tissue is markedly altered, such as leptin-deficient lipodystrophy, plasma levels of ANGPTL3 have been found to be markedly elevated [15]. At the same time, leptin replacement therapy with Metreleptin produced a significant decrease in plasma ANGPTL3 and TG levels [15]. Of note, some studies have shown that ANGPTL3 can be involved in carbohydrate metabolism [16,17], but the relationship of ANGPTL3 and 4 with parameters of glucose metabolism has not been firmly established. Since several drugs inhibiting ANGPTL3 are now becoming available [18], clinical studies considering the variations of ANGPTLs in extreme metabolic conditions are of uttermost importance in order to clarify the interplay between ANGPTLs and the flux of energy substrates, with aim to give the best indication for future available ANGPTL3 inhibition. We have chosen bariatric surgery (BS) patients as a model of profound metabolic changes in adipose tissue [19], as well as in hormonal status [20] and energy substrate metabolism [21]. The aim of the study was to gain information on the relationship between adipose tissue, energy balance and ANGPTLs regulation. We measured ANGPTL3 and ANGPTL4 levels in patients with obesity and diabetes before and after BS. By this approach, we intended to verify whether: 1) the reduction of adipose mass caused by BS may lead to changes in circulating ANGPTLs and 2) these effects are dependent on the type of BS and may be related to some parameters of glucose and lipid metabolism.
2. Methods
2.1. Study patients
The study group included 45 patients eligible for BS due to morbid obesity and/or type two diabetes (T2D). The baseline clinical characteristics of these patients have been partially described in a previous report [22].
In brief, 27 patients with morbid obesity (BMI range 38–62 kg/m2) underwent RYGB; of them, 11 had normal glucose tolerance (NGT) and 16 had T2D (Obese T2D group). Eighteen, patients with non-morbid obesity (BMI range 23–33 kg/m2) and decompensated T2D (non-Obese T2D) underwent BPD. Assignment to RYGB or BPD was based on the decision of the treating physician. In non-T2D patients, glucose status was determined by an oral glucose tolerance test (OGTT) according to standard procedures [23,24]. In the BPD group, T2D patients were treated with oral glucose-lowering agents and/or insulin (metformin in 6 patients, metformin plus sulfonylurea in 3 patients, and metformin plus insulin in 6 patients). In the RYGB group, 6 T2D patients were treated with diet alone and 10 were on oral glucose-lowering agents and/or insulin (metformin in 6 patients and metformin plus sulfonylurea in 3 patients and metformin plus insulin in 1 patient).
Study subjects were studied before surgery (baseline) after an overnight fast by measuring biochemical and hormonal parameters as well as by performing a euglycemic-hyperinsulinemic clamp, as described below. All patients in the RYGB group and 15 patients in the BPD group were restudied 1-year after surgery with the same protocol.
The study protocol was approved by the Ethic Committee of the University of Pisa. The nature and purpose of the study were carefully explained to all participants. All subjects were asked to voluntarily participate into the present study and written informed consent was obtained from all participants, research and clinical practices were carried out in accordance with the principles of the Helsinki Declaration of 1964 and its revision of 2018.
2.2. Euglycemic-hyperinsulinemic clamp
All investigations were performed in the morning after an overnight fast. Patients with T2D were asked to stop metformin 1 week and sulfonylureas 48–72 h before the study; in those on insulin, injections were discontinued 16 h before the metabolic study (patients on bedtime glargine had been switched to NPH 2 days before the study). The euglycemic-hyperinsulinaemic clamp was performed as already described [23]. In brief, a catheter was inserted into an antecubital vein for insulin and glucose infusion and another catheter was inserted retrogradely into a vein on the dorsum of the hand for blood drawing. The hand was heated to obtain arterialization of venous blood. At time −20, −10, and 0 min, blood samples were obtained from the arterialized vein for the measurement of glucose and insulin. At time 0, a primed-continuous insulin (Humulin R; Eli Lilly and Company, Indianapolis, USA) infusion (at a rate of 240 pmol min−1·m−2) was started and continued for 180 min; plasma glucose levels were measured every 5 min throughout the clamp. Plasma insulin concentrations were measured every 20 min between 120 and 180 min after the start of insulin infusion. Fat-free mass (FFM) was estimated by electrical bioimpedance (TBF 300; Tanita, Tokyo, Japan).
Insulin-stimulated glucose disposal (M value) was calculated as the mean exogenous glucose infusion rate during the last 40 min of the clamp corrected for changes in glucose concentration within a distribution volume of 200 mL per kilogram of body weight. M was expressed per kilogram of FFM (in umol·min−1·kgFFM−1) and used to estimate the insulin sensitivity [20].
2.3. Analytical methods
Blood samples were drawn in standard fasting conditions (at least 10 h after the last meal) and then separated by centrifugation at 3000×g for 15 min and stored at −80 °C until assays. Plasma glucose was measured by the glucose oxidase technique on a Beckman Glucose Analyzer (Beckman, Fullerton, CA). Plasma insulin was assayed by a specific radioimmunoassay (Linco Research, St. Charles, MO, and MYRIA, Technogenetics, Milan, Italy, respectively). Plasma FFA concentrations were measured spectrophotometrically by an automated procedure (Synchro CX4; Beckman, Brea, CA, USA). Plasma triglycerides and serum high-density lipoprotein (HDL) cholesterol were assayed in duplicate by standard spectrophotometric methods on a Synchron Clinical System CX4 (Beckman Instruments, Fullerton, USA). Total bile acids (TBA) were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Waters Quattro Micro with Waters 2795 Alliance HPLC). Serum ANGPTL3 and ANGPTL4 levels were measured by sandwich enzyme immunoassays using commercial ELISA kits (RND systems #DANL30, and Biovendor #RD191073200R). The assay was performed according to the manufacturer’s protocol; sensitivity values were 0.035 ng/mL for ANGPTL3 and 0.173 ng/mL for ANGPTL4. Intra- and inter-assay coefficients of variation for ANGPTL3 and ANGPTL4 measurements were 3% and 8%, respectively. All measurements were carried out in the same run to minimize intra-assay variability.
2.4. Statistical analysis
Data are reported as mean ± SE or median [interquartile range] for variables with non-normal distribution. Group differences were tested by Mann–Whitney U test and Wilcoxon signed rank test. For selected variables, two-way ANOVA for repeated measures was used to compare surgical operations across time of study. Simple associations were tested by calculating Spearman’s rank correlation coefficient (ρ), data fitting was performed by non-linear regression techniques. Multivariate analysis was carried out using general linear models; results are given as the standardized regression coefficient. Statistical analyses were performed using Stat View 5.0 software, a p value ≤ 0.05 was considered significant.
3. Results
3.1. Baseline characteristics
The baseline characteristics of the study participants, grouped according to obesity and type 2 diabetes (T2D) status are summarized in Table 1. As compared with patients with obesity and T2D, the non-obese T2D subjects were older and leaner, and showed higher fasting glucose, FFA, and triglyceride concentrations but lower fasting insulin and HDL-cholesterol levels. Insulin sensitivity (M value) and circulating levels of ANGPTL3 and ANGPTL4 did not differ significantly across groups (Table 1). No differences in the ANGPTL3 and ANGPTL4 plasma levels were observed according to the sex in the pooled data or in single groups analyzed separately (p = NS for all). In the pooled data from all participants, baseline ANGPTL3 levels were positively related to M value (r = 0.37, p = 0.02), whereas baseline ANGPTL4 levels were inversely related to M value (r = −0.37, p = 0.03).
Table 1.
Baseline characteristics of the study subjects.
| Obese NGT |
Obese T2D |
non-Obese T2D |
|
|---|---|---|---|
| Number (F/M) | 11 (10/1) | 16 (11/5) | 18 (7/11) |
| Age (years) | 43 ± 2 | 50 ± 2§ | 56 ± 1* |
| Body weight (kg) | 145 ± 6 | 132 ± 7 | 78 ± 2* |
| BMI (kg·m−2) | 52.7 ± 1.9 | 49.8 ± 2.1 | 28.2 ± 0.6* |
| Fasting glucose (mmol/L) | 5.2 ± 0.1 | 7.2 ± 0.6§ | 12.1 ± 0.4* |
| Fasting insulin (pmol/L) | 138 [112] | 126 [112] | 64 [45]* |
| FFA (mEq/L) | 0.60 ± 0.05 | 0.70 ± 0.04 | 0.84 ± 0.04* |
| Total cholesterol (mmol/L) | 4.34 ± 0.18 | 4.44 ± 0.30 | 5.31 ± 0.39 |
| Triglycerides (mmol/L | 1.13 ± 0.15 | 1.73 ± 0.46 | 1.99 ± 0.16* |
| HDL-cholesterol (mmol/L) | 1.23 ± 0.12 | 0.98 ± 0.07 | 1.17 ± 0.07* |
| LDL-cholesterol (mmol/L) | 2.61 ± 0.17 | 2.70 ± 0.24 | 3.25 ± 0.34 |
| TBA (μmol/L) | 1.62 [3.06] | 2.57 [2.76] | 1.65 [1.62] |
| M value (μmol·min−1·kgFFM−1) | 22.1 [13.2] | 18.1 [22.4] | 20.6 [3.8] |
| ANGPTL3 (ng/ml) | 256 ± 20 | 275 ± 23 | 225 ± 17 |
| ANGPTL4 (ng/ml) | 27.0 ± 0.8 | 25.2 ± 1.0 | 27.2 ± 1.0 |
Data is given as mean ± SEM or median [interquartile range]. NGT normal glucose tolerance; T2D, type 2 diabetes; BMI, body mass index; FFA, free fatty acids; HDL, high density lipoprotein; LDL, low density lipoprotein; TBA, total bile acid; M value, insulin sensitivity by the euglycemic insulin clamp; ANGPTL3, angiopoietin like protein 3; ANGPTL4, angiopoietin like protein 4.
p ≤ 0.05 obese NGT vs obese T2D
p ≤ 0.05 obese T2D vs non-Obese T2D.
3.2. After surgery changes
Table 2 compares anthropometric and metabolic parameter before and 1 year after BS. Regardless of the BS procedure used, all patients achieved significant BMI, fasting glucose and insulin reduction, together with an improvement in total and LDL cholesterol levels. Only Roux-en-Y gastric bypass (RYGB) patients, however, showed a significant increase in HDL-C and a reduction in serum TG. Both BS techniques were associated with a significant increase in total bile acids (TBA), which was particularly pronounced in subjects undergoing BPD. The patients in the latter group also experienced a decrease in FFA concentrations (p = 0.009). As expected, both RYGB and BPD resulted in a marked improvement in insulin sensitivity (M-value).
Table 2.
Anthropometric and laboratory parameters pre-surgery vs 1-year post surgery.
| RYGB |
BPD |
|||||
|---|---|---|---|---|---|---|
| Pre-surgery | Post-surgery | p | Pre-surgery | Post-surgery | p | |
| N | 27 | 27 | – | 15 | 15 | – |
| Age (years) | 47 ± 2 | – | – | 57 ± 1§ | – | – |
| Weight (kg) | 137 ± 5 | 93 ± 4 | <0.0001 | 78 ± 3§ | 64 ± 2* | 0.0007 |
| BMI (kg·m−2) | 50.9 ± 1.5 | 34.5 ± 1.1 | <0.0001 | 28.2 ± 0.7§ | 23.2 ± 0.7* | 0.0007 |
| Fasting glucose (mmol/L) | 6.4 ± 0.4 | 5.0 ± 0.1 | <0.0001 | 12.3 ± 0.5§ | 7.3 ± 0.4* | 0.0007 |
| Fasting insulin (pmol/L) | 137 [120] | 44 [22] | <0.0001 | 61 [52] § | 34 [9] | 0.0007 |
| FFA (mEq/L) | 0.68 [0.22] | 0.62 [0.18] | ns | 0.85 [0.24] | 0.80 [0.27] | 0.009 |
| Total cholesterol (mmol/L) | 4.40 ± 0.19 | 4.04 ± 0.11 | 0.02 | 5.28 ± 0.42 | 3.52 ± 0.12* | 0.009 |
| Triglycerides (mmol/L) | 1.48 ± 0.27 | 0.99 ± 0.07 | 0.002 | 1.99 ± 0.18 | 1.96 ± 0.27* | ns |
| HDL-cholesterol (mmol/L) | 1.09 ± 0.07 | 1.27 ± 0.07 | 0.02 | 1.18 ± 0.07 | 1.06 ± 0.07* | ns |
| LDL-cholesterol (mmol/L) | 2.66 ± 0.15 | 2.33 ± 0.08 | 0.03 | 3.21 ± 0.36 | 1.59 ± 0.07* | 0.006 |
| TBA (μmol/L) | 2.36 [3.02] | 4.30 [6.14] | 0.005 | 1.55 [1.93] | 7.11 [8.03] | 0.002 |
| M value (μmol·min−1·kgFFM −1) | 19.8 [19.0] | 41.9 [10.3] | <0.0001 | 19.4 [2.7] | 33.9 [9.1]* | 0.0007 |
Data is given as mean ± SEM or median [interquartile range]. RYGB, Roux-en-Y gastric bypass; BPD, biliopancreatic diversion; BMI, body mass index; FFA, free fatty acids; HDL, high density lipoprotein; LDL, low density lipoprotein; TBA, total bile acids; M value, insulin sensitivity by the euglycemic insulin clamp; p post-surgery vs Pre-surgery
p ≤ 0.05 BPD vs RYGB pre-surgery
p ≤ 0.05 BPD vs RYGB post-surgery.
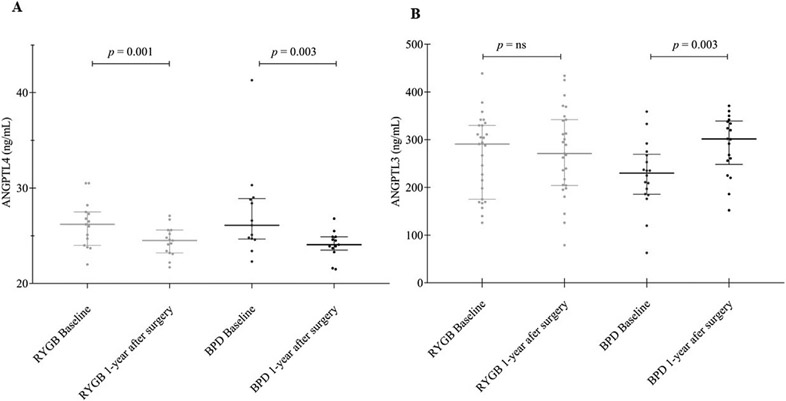
ANGPTL4 levels were significantly decreased in both groups: from 26.6 ± 0.6 to 24.4 ± 0.3 ng/mL (p = 0.001) in the RYGB and from 27.9 ± 1.5 to 24.0 ± 0.5 ng/mL (p = 0.003) in the BPD group (Fig. 1A). In contrast, ANGPTL3 levels did not show any changes in RYGB patients (from 256 ± 20 to 228 ± 28 ng/mL; p = ns), whereas it was significantly increased (from 225 ± 20 to 300 ± 15 ng/mL, p = 0.003) in BPD-treated patients (Fig. 1B). To exclude any potential sexual dimorphism in the change of serum ANGPTL3 and ANGPTL4 after surgery, we analyzed the data by repeated-measures ANOVA. We observed that the change in ANGPTL4 after surgery (p = 0.0001) was influenced neither by kind of surgery nor by sex, whereas the change in ANGPTL3 (p = 0.03) was influenced by surgical technique (p = 0.02), but not by sex.
Figure 1.
ANGPTL4 and ANGPTL3 variation in RYGB and BPD surgeries. Box plot of ANGPTL4 (A) and ANGPTL3 (B) levels in Roux-en-Y Gastric Bypass (RYGB) surgery and Biliopancreatic Diversion (BPD).
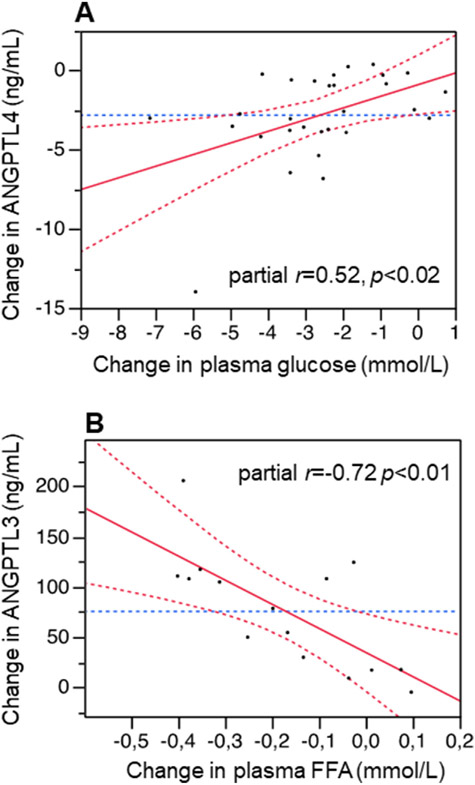
Table 3 shows a multiple regression model for ANGPTL4 variation after bariatric surgery. As both RYGB and BPD patients showed a reduction in ANGPTL4 levels 1-year after surgery, data from both groups were pooled together in this model. After adjusting for age, and for BMI and M value changes, the main determinant of ANGPTL4 changes after surgery was plasma glucose variation. Changes in plasma glucose explained 58% of ANGPTL4 reduction after BS (R2 = 0.278; p = 0.06) (Table 3, Fig. 2A).
Table 3.
Δ-ANGPTL4 multiple regression analysis.
| Δ-ANGPTL4 |
||||
|---|---|---|---|---|
| β | Std β | t-value | p | |
| Δ-Glucose | 0.736 | 0.586 | 2.561 | 0.0169 |
| Δ-M value | 0.115 | 0.366 | 1.755 | 0.0916 |
| Δ-BMI | 0.091 | 0.233 | 0.978 | 0.3375 |
Multivariate regression expressing changes in ANGPTL4 concentrations (Δ-ANGPTL4) in the combined surgery group.
Figure 2.
Association analysis of ANGPTL4 and ANGPTL3 variation with metabolic parameters. A. Association of changes in plasma ANGPTL4 (leveraged for the concomitant changes in M changes and BMI changes by multiple regression) with changes in plasma glucose concentrations; B. Association of changes in plasma ANGPTL3 (leveraged for age, concomitant changes in M and total bile acids by multiple regression) with changes in plasma FFA concentrations.
In simple regression analysis combined data pre- and post-surgery in the BPD group, we found ANGPTL3 levels correlates directly with age (r = 0.36 p = 0.04), serum TBA (r = 0.40 p = 0.02) and M value (r = 0.61; p = 0.0001) and inversely with serum FFA (r = −0.42 p = 0.01). In a multiple regression model including age, and surgery-induced changes in M value, change in FFA, and change in TBA, the decrease in FFA and the increase of M were the principal determinants of the ANGPTL3 increase after BPD (Table 4, Fig. 2B). This result was confirmed in stepwise regression, in which variation of FFA and M-value were the first and second variables forced to enter into the model (p = 0.0005; F value 23.2 for FFA, F value 4.2 for M-value variation). In the proposed model, after correction for age and TBA variation, changes in FFA and insulin sensitivity explains respectively 61% and 35% of ANGPTL3 increase after BPD (R2 = 0.781; p = 0.0024) (Table 4).
Table 4.
Δ-ANGPTL3 multiple regression analysis.
| Δ-ANGPTL3 |
||||
|---|---|---|---|---|
| β | Std β | t-value | p | |
| Δ-FFA | −239 | 0.608 | −3.304 | 0.0080 |
| Δ-M value | 4.533 | 0.350 | 2.321 | 0.0427 |
| Δ-TBA | 3.659 | 0.261 | 1.445 | 0.1790 |
| Age | −2.219 | 0.103 | 0.679 | 0.5123 |
Multivariate regression expressing changes in ANGPTL3 concentrations (Δ-ANGPTL3) in BPD patients.
4. Discussion
To the best of our knowledge, this is the first study investigating the long-term effect of BS on plasma concentrations of ANGPTL3 and ANGPTL4. We included both NGT and T2D patients with obesity to gauge the effect of surgery over a broader range of insulin/glucose abnormalities and considered both RYGB and BPD to evaluate the differential effect of these procedures on ANGPTLs.
The main findings of this study are: a) both BPD and RYGB cause a reduction in ANGPTL4 levels; b) ANGPTL3 increases after BPD but not after RYGB; c) the increase in ANGPTL3 after BPD is significantly associated with the concomitant reduction in FFA. In addition, we found that NGT individuals with morbid obesity have similar levels of ANGPTL3 and ANGPTL4 as T2D patients with or without obesity, and that insulin sensitivity is directly related to ANGPTL3 and inversely related to ANGPTL4.
As expected, BS procedures effectively reduced body weight in all patients, and this paralleled a near doubling in insulin sensitivity. It is noteworthy that TG reduction was obtained with RYGB but not with BPD, which conversely was more effective in reducing TC and LDL-C. As described in a recent meta-analysis, RYGB is the gold standard surgery technique in morbid obesity, but BPD is more effective for the long-term remission of T2D and cholesterol reduction [25,26]. The latter finding derives from the fact that BPD is a malabsorptive procedure, which causes enhanced bile acid loss. This in turn stimulates bile acid synthesis and enhanced liver uptake of circulating LDL [22].
In the fasted condition, plasma levels of ANGPTL4 were found to be significantly reduced in all patients treated with either RYGB or BPD. The estimated overall size of this reduction was ~12%. Since the adipose tissue is an important site of ANGPTL4 production [2,27], this reduction can be easily explained by the marked post-surgical reduction of body fat mass. In line with this result, patients with obesity following a hypocaloric diet for 2 years also exhibited a decrease in serum ANGPTL4 in parallel to the decreased fat mass [28]. Insulin is a negative regulator of ANGPTL4 synthesis and secretion through the inhibition of peroxisome-proliferator receptor α and γ (PPAR-α and PPAR-γ), which are the main inducers of this angiopoietin [2,29]. The observed reduction in circulating ANGPTL4 may be ascribed, at least in part, to the post-surgical improvement of whole-body insulin sensitivity with the attendant fall in glucose and insulin levels (Tables 2 and 3). As ANGPTL4 acts as an inhibitor of LPL activity, its reduction following BS might activate TG hydrolysis, thereby leading to TG lowering. This hypothesis was recently proved by Singh et al. [30] that reported, in a mouse model selectively knocked-out for ANGPTL4, a consistent reduction of circulating lipids, increased hepatic clearance of dietary derived chylomicrons and increased liver and adipose tissue insulin sensitivity [30]. Unfortunately, our study did not include measurements of post-heparin lipase activity (PHLA) before and after surgery so that further studies are needed to clarify this point.
The effect of BS on ANGPTL3 appears to be strictly dependent on the surgical procedure, as its levels were unchanged by RYGB but increased significantly after BPD. We didn’t measure the ANGPTLs early after surgery, so that we cannot exclude a time-dependent change of ANGPTL3 after surgery as described for ANGPTL8 after sleeve gastrectomy in patients with diabetes [31]. Although several studies reported ANGPTL3 to be directly related to BMI (with higher levels in people with obesity), the positive relationship between body fat and ANGPTL3 has not been consistently confirmed [13,14]. We did not find a significant correlation between ANGPTL3 and TG levels, which is in line with some previously reported results [32] but not with others [33]. This controversial relationship suggests that other factors might affect their mutual regulation. In a previous study, we reported that the relation between plasma levels of ANGPTL3 and TG is not linear [32]. In fact, TG levels (and other lipids such as total cholesterol, HDL- and LDL-cholesterol) were strongly correlated with ANGPTL3 only when ANGPTL3 levels are below 60 ng/dL, whereas at higher levels there was no longer a significant association with serum lipids [32].
The relation between ANGPTL3, insulin and glucose is still debated. Haridas et al. reported a reduction in plasma ANGPTL3 during a euglycemic hyperinsulinemic clamp in healthy subjects, and in culture medium of IHH cells treated with insulin [17]. Yilmaz et al. found a direct relationship between HOMA-IR (an indirect index of hepatic insulin resistance) and plasma ANGPTL3 in patients with fatty-liver disease [16]. In contrast, Schmid at al. found no differences in ANGPTL3 levels in subjects during OGTT [34].
In our patients, the BPD-induced rise in ANGPTL3 was directly related to changes in insulin sensitivity and inversely related to changes in serum FFA [11]. In BPD patients the reduction of FFA 1-year after surgery could be explained by both adipocyte mass reduction and the improvement in insulin sensitivity. The post-surgical doubling of insulin sensitivity would raise intracellular glucose concentrations, and activate LXR thereby stimulating lipogenesis and liver lipid export [35-37]. In these subjects, the concomitant reduction in cholesterol and bile acids is a double hit for LXR hyperactivation, thereby promoting ANGPTL3 secretion. In support to our hypothesis, Cinkajzlová et al. [14] found increased levels of serum ANGPTL3 in patients suffering from short-bowel syndrome (SBS) in comparison with healthy controls [14]. Notably, BPD patients can be considered as analog to SBS patients in terms of nutrients absorption and loss.
In interpreting these results, several limitations must be considered. The number of patients included in the study is small and the assignment of patients to RYGB or BPD was not randomized (due to partially different indications and clinical experience with the two procedures). More importantly, the difference in BMI among RYGB and BPD patients is wide and glucose control is very different in the two surgical groups of diabetic patients. Finally, plasma levels of ANGPTL8, another angiopoietin-like protein involved in the activation of ANGPTL3, were not investigated.
In summary, BS showed the expected effect in improving the metabolic status of obese patients with or without T2D. While circulating ANGPTL4 was uniformly decreased by surgery, ANGPTL3 remained stable in RYGB patients but increased in BPD patients. This suggests that the chronic nutrient loss and malabsorption associated with BPD may be counterbalanced by the increased production of ANGPTL3 in the liver. It is possible that the decrease in ANGPTL4 (expressed mostly in WAT) may promote the uptake and storage of TG-derived lipids into the WAT, whereas the increase in ANGPTL3 (expressed mostly in the liver) may potentially slow down lipid uptake into the liver. In this regard, it is worth mentioning that our results highlighted a relationship between FFA, bile acid metabolism and insulin sensitivity with ANGPTL3, thus opening a new view on the complex regulation of ANGPTL3 metabolism. Due to the limited number of subjects involved and the non-randomized non-controlled nature of this study further in vitro and in vivo studies are necessary to detail the molecular mechanism underlying this relationship.
Acknowledgements
SB contributed to this paper as part of his Ph.D. studies in Biotechnologies and Clinical Medicine at “Sapienza” University of Rome.
Funding
This research has been partially supported by funds from Sapienza University of Rome, Italy (#000106-19-Arca); the National Institutes of Health, USA (#R01HL12564 and #R01HL125649); and a grant from Italian Ministry of Health, Italy (RF-2011-02348446).
Footnotes
Declaration of competing interest
LD has received personal fees for public speaking, consultancy or grant support from Amryt Pharmaceuticals, Akcea Therapeutics, Pfizer, Amgen and Sanofi; MA has received research grant support from Amryt Pharmaceutical, Amgen, IONIS, Akcea Therapeutics, Pfizer and Sanofi; has served as a consultant for Amgen, Aegerion, Akcea Therapeutics, Regeneron, Sanofi and Alfasigma and received lecturing fees from Amgen, Amryth Pharmaceutical, Pfizer, Sanofi and AlfaSigma. EF reports receiving consultancy/speaker fees, outside the present work, from Boehringer Ingelheim, Lilly&Co., AstraZeneca, and Sanofi. Other authors have declared no conflict of interest.
References
- [1].Zhang R, Zhang K. An updated ANGPTL3-4-8 model as a mechanism of triglyceride partitioning between fat and oxidative tissues. Prog Lipid Res 2021;85. 10.1016/J.PLIPRES.2021.101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bini S, D’Erasmo L, Di Costanzo A, Minicocci I, Pecce V, Area M. The interplay between angiopoietin-like proteins and adipose tissue: another piece of the relationship between adiposopathy and cardiometabolic diseases? Int J Mol Sci 2021;22(2):1–16. 10.3390/ijms22020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci U S A 2006;103(46):17450–5. 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Inaba T, Matsuda M, Shimamura M, Takei N, Terasaka N, Ando N, et al. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J Biol Chem 2003;278(24):21344–51. 10.1074/jbc.M213202200. [DOI] [PubMed] [Google Scholar]
- [5].Haller JF, Mintah IJ, Shihanian LM, Stevis P, Buckler D, Alexa-Braun CA, et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res 2017;58(6):1166–73. 10.1194/jlr.M075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perdomo CM, Gómez-Ambrosi J, Becerril S, Valentí V, Moncada R, Fernández-Sáez EM, et al. Role of ANGPTL8 in NAFLD improvement after bariatric surgery in experimental and human obesity. Int J Mol Sci 2021;22(23):12945. 10.3390/ijms222312945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen YQ Pottanat TG, Siegel RW, Ehsani M, Qian Y-WW, Zhen EY, et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res 2020;61(317). 10.1194/jlr.ra120000781.jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Röster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, et al. Transgenic angiopoietin-like (Angptl)4 overexpression and targeted disruption of Angptl4 and Angptl3: regulation of triglyceride metabolism. Endocrinology 2005;146(11):4943–50. 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- [9].Minicocci I, Tikka A, Poggiogalle E, Metso J, Montali A, Ceci F, et al. Effects of angiopoietin-like protein 3 deficiency on postprandial lipid and lipoprotein metabolism. J Lipid Res 2016;57(6):1097–107. 10.1194/jlr.P066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Di Costanzo A, Di Leo E, Noto D, Cefalù AB, Minicocci I, Polito L, et al. Clinical and biochemical characteristics of individuals with low cholesterol syndromes: a comparison between familial hypobetalipoproteinemia and familial combined hypolipidemia. J Clin Lipidol 2017;11(5):1234–42. 10.1016/j.jacl.2017.06.013. [DOI] [PubMed] [Google Scholar]
- [11].Bini S, Pecce V, Di Costanzo A, Polito L, Ghadiri A, Minicocci I, et al. The fibrinogen-like domain of ANGPTL3 facilitates lipolysis in 3T3-L1 cells by activating the intracellular erk pathway. Biomolecules 2022;12(4):585. 10.3390/biom12040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barja-Fernández S, Folgueira C, Castelao C, Pena-León V, González-Saenz P, Vázquez-Cobela R, et al. ANGPTL-4 is associated with obesity and lipid profile in children and adolescents. Nutrients 2019;11(6). 10.3390/nu11061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garcés MF, Buell-Acosta JD, Rodríguez-Navarro HA, Pulido-Sánchez E, Rincon-Ramírez JJ, Moreno-Ordóñez DC, et al. Serum angiopoietin-like 3 levels are elevated in obese non diabetic men but are unaffected during an oral glucose tolerance test. Sci Rep 2020;10(1). 10.1038/s41598-020-77961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cinkajzlová A, Mráz M, Lacinová Z, Kloučková J, Kaválková P, Kratochvílová H, et al. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: the effect of weight reduction and realimentation. Nutr Diabetes 2018;8(1). 10.1038/s41387-018-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muniyappa R, Abel BS, Asthana A, Walter MF, Cochran EK, Remaley AT, et al. Metreleptin therapy lowers plasma angiopoietin-like protein 3 in patients with generalized lipodystrophy. J Clin Lipidol 2017;11(2):543–50. 10.1016/j.jacl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yilmaz Y, Ulukaya E, Atug O, Dolar E. Serum concentrations of human angiopoietin-like protein 3 in patients with nonalcoholic fatty liver disease: association with insulin resistance. Eur J Gastroenterol Hepatol 2009;21(11):1247–51. 10.1097/MEG.0b013e32832b77ae. [DOI] [PubMed] [Google Scholar]
- [17].Haridas PAN, Soronen J, Sädevirta S, Mysore R, Quagliarini F, Pasternack A, et al. Regulation of angiopoietin-like proteins (ANGPTLs) 3 and 8 by insulin. J Clin Endocrinol Metab 2015;100(10):E1299–307. 10.1210/jc.2015-1254. [DOI] [PubMed] [Google Scholar]
- [18].D’Erasmo L, Bini S, Area M. Rare treatments for rare dyslipidemias: new perspectives in the treatment of homozygous familial hypercholesterolemia (HoFH) and familial chylomicronemia syndrome (FCS). Curr Atherosclerosis Rep 2021;23(11):65. 10.1007/s11883-021-00967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Camastra S, Vitali A, Anselmino M, Gastaldelli A, Bellini R, Berta R, et al. Muscle and adipose tissue morphology, insulin sensitivity and beta-cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery. Sci Rep 2017;7(1):9007. 10.1038/s41598-017-08444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Camastra S, Manco M, Frascerra S, Iaconelli A, Mingrone G, Ferrannini E. Daylong pituitary hormones in morbid obesity: effects ofbariatric surgery. Int J Obes 2009;33(1):166–72. 10.1038/ijo.2008.226. [DOI] [PubMed] [Google Scholar]
- [21].Camastra S, Palumbo M, Santini F. Nutrients handling after bariatric surgery, the role of gastrointestinal adaptation. Eat Weight Disord - Stud Anorexia, Bulim Obes April 2021. 10.1007/S40519-021-01194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferrannini E, Camastra S, Astiarraga B, Nannipieri M, Castro-Perez J, Xie D, et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes 2015;64. 10.2337/db15-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54(8):2093–102. 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- [24].Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(SUPPL. 1):S62–9. 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ding L, Fan Y, Li H, Zhang Y, Qi D, Tang S, et al. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials. Obes Rev 2020;21(8). 10.1111/obr.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu G, Song M. Recent advances in the mechanisms underlying the beneficial effects of bariatric and metabolic surgery. Surg Obes Relat Dis 2021;17(1):231–8. 10.1016/j.soard.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MKC, et al. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife 2015;4(OCTOBER2015). 10.7554/eLife.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barja-Fernandez S, Moreno-Navarrete JM, Folgueira C, Xifra G, Sabater M, Castelao C, et al. Plasma ANGPTL-4 is associated with obesity and glucose tolerance: cross-sectional and longitudinal findings. Mol Nutr Food Res 2018;62(10):e1800060. 10.1002/mnfr.201800060. [DOI] [PubMed] [Google Scholar]
- [29].Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 2000;275(37):28488–93. 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- [30].Singh AK, Chaube B, Zhang X, Sun J, Citrin KM, Canfrán-Duque A, et al. Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. J Clin Invest 2021;131(17). 10.1172/JCI140989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Al-Shawaf E, Al-Ozairi E, Al-Asfar F, Al-Beloushi S, Kumari S, Tuomilehto J, et al. Biphasic changes in angiopoietin-like 8 level after laparoscopic sleeve gastrectomy and type 2 diabetes remission during a 1-year follow-up. Surg Obes Relat Dis 2018;14(9):1284–94. 10.1016/j.soard.2018.05.026. [DOI] [PubMed] [Google Scholar]
- [32].Fazio S, Minnier J, Shapiro MD, Tsimikas S, Tarugi P, Averna MR, et al. Threshold effects of circulating angiopoietin-like 3 levels on plasma lipoproteins. J Clin Endocrinol Metab 2017;102(9):3340–8. 10.1210/jc.2016-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li J, Yang Y, Jiao X, Yu H, Du Y, Zhang M, et al. The clinical role of angiopoietin-like protein 3 in evaluating coronary artery disease in patients with obstructive sleep apnea. Cardiovasc Drugs Ther 2020;34(6):773–80. 10.1007/s10557-020-06991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schmid A, Belikan H, Höpfinger A, Schäffler A, Karrasch T. Impact of oral lipid and glucose tolerance tests on the postprandial concentrations of angiopoietin-like proteins (Angptl) 3 and 4. Eur J Nutr. December 2021:1–11. 10.1007/S00394-021-02748-0/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol 2018;14(8):452–63. 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cariello M, Piccinin E, Moschetta A. Transcriptional regulation of metabolic pathways via lipid-sensing nuclear receptors. Cell Mol Gastroenterol Hepatol. February 2021. 10.1016/j.jcmgh.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iizuka K, Takao K, Kato T, Horikawa Y, Takeda J. ChREBP reciprocally regulates liver and plasma triacylglycerol levels in different manners. Nutrients 2018;10(11):1699. 10.3390/nu10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]