FIGURE 3.
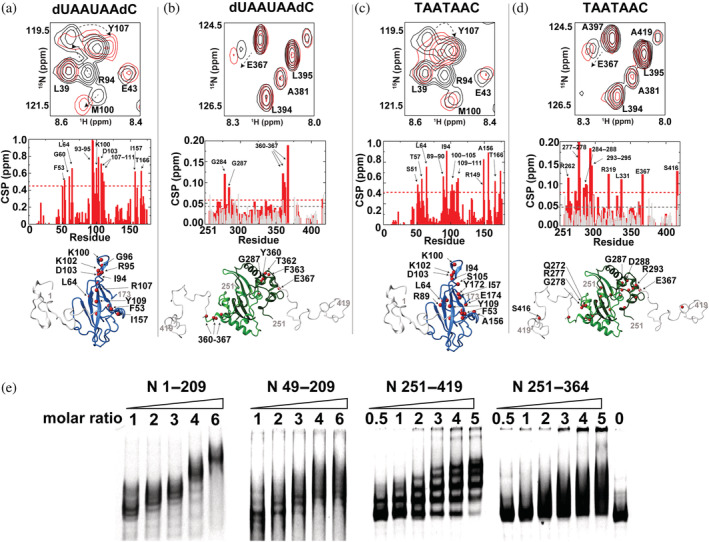
Nuclear magnetic resonance titration comparison of the N‐terminal and C‐terminal regions of the N protein with RNA and DNA fragments. Each sample comprised 100 μM 15 N‐labeled N 1–209, 200 μM N 251–419. (a) N 1–209 binding to dUAAUAAdC. Top: Section of 15 N‐HSQC of 100 μM N 1–209 alone (black) and with 400 μM dUAAUAAdC (red). Middle: Global chemical shift comparisons (CSPs) between these two spectra. Bottom: Amides that induce greater than the average plus 1 standard deviation (red spheres) are mapped onto the structure of the N‐terminal domain (NTD) with modeled flanking regions. (b) N 251–419 dimerization domain binding to dUAAUAAdC. Top: Section of 15 N‐HSQC of 200 μM N 251–419 alone (black) and with 400 μM dUAAUAAdC (red). Middle: Global CSPs between these two spectra (red) along with a dilution of free N 251–419 alone at 200 and 100 μM (gray). Bottom: Amides that induce greater than the average plus 1 standard deviation (red spheres) are mapped onto the structure of the C‐terminal domain (CTD) with modeled flanking regions. (c) N 1–209 binding to TAATAAC. Top: Section of 15 N‐HSQC of 100 μM N 1–209 alone (black) and with 400 μM TAATAAC (red). Middle: Global CSPs between these two spectra. Bottom: Amides that induce greater than the average plus 1 standard deviation (red spheres) are mapped onto the structure of the NTD with modeled flanking regions. (d) N 251–419 dimerization domain binding to TAATAAC. Top: Section of 15 N‐HSQC of 200 μM N 251–419 alone (black) and with 400 μM TAATAAC (red). Middle: Global CSPs between these two spectra (red) along with a dilution of free N 251–419 at 200 and 100 μM (gray). Bottom: Amides that induce greater than the average plus 1 standard deviation (red spheres) are mapped onto the structure of the CTD with modeled flanking regions. (e) Electrophoresis mobility shift assay performed with 5′ untranslated region n.a. 80–294 with increasing concentration of four different N protein constructs: N 1–209, N 49–209, N 251–419, and N 251–364. All gels comprised of 10% acrylamide with 2 mM MgCl2 and are stained with ethidium bromide.
