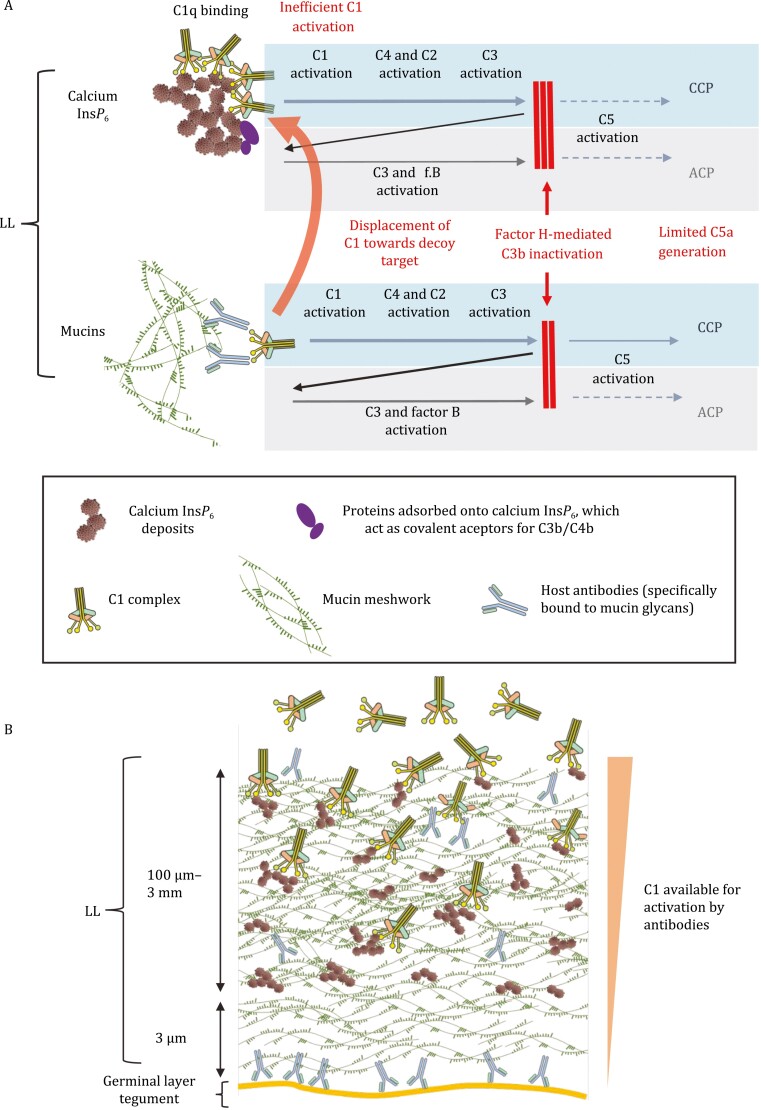
Figure 2.
Proposed model summarizing complement activation and control on the LL and the hydatid wall. (A) Although the lectin pathway is not activated by the LL, the structure interacts both with the classical (CCP) and the alternative complement pathways (ACP). Calcium Insp6 in the LL binds C1 complex through its C1q component. Although inefficiently, this causes C1 activation and initiates the CCP. This results in deposition of the activated forms of the CCP component C4 (C4b) and of the central component C3 (C3b) on calcium Insp6 itself. As Insp6 has no covalent acceptors for C4/C3, this deposition is bridged by adsorbed serum proteins. C3b deposited on calcium Insp6 is very rapidly inactivated in factor H-dependent fashion, which also means that ACP activation is curtailed. C1 binding to antibodies bound to the mucins is reduced as a consequence of the abundant calcium Insp6 acting as a decoy target for C1. Any remaining CCP activation on the mucins, as well as ACP activation, is curtailed as a consequence of factor H having affinity for (unknown sites in) the mucins. Overall, calcium Insp6 re-directs CCP activation from the mucin-bound antibodies onto itself, without either enhancing or reducing C5 activation on the LL. (B) The high binding capacity of calcium Insp6 deposits for C1q, combined with a restriction to the access of C1 imposed by the LL mucin gel would prevent the binding of functional C1 to the germinal layer tegument. This would avoid CCP activation triggered by antibodies bound to the germinal layer. In other words, the re-directioning of CCP activation onto calcium Insp6, although “neutral” when considering C5 activation by the LL only, would have a parasite-protective role when the germinal layer is also considered. The previously reported absence of calcium Insp6 deposits in the innermost 3 µm of the LL would prevent bystander damage to the germinal layer tegument by activation CCP initiated on calcium Insp6. Part (B) has been reproduced from reference (Barrios et al., 2019) (published by Elsevier).