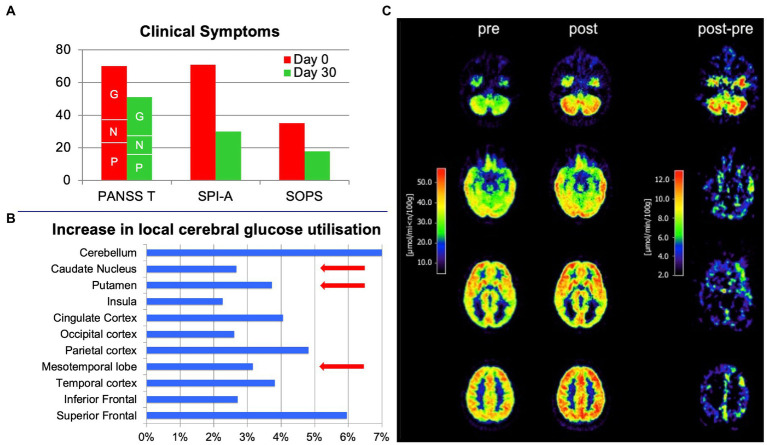
Figure 2.
Effects of treatment with 600 mg oral cannabidiol per day in a 19-year-old male with clinically high mental risk before (pre) and on day 30 (post). (A) Positive and Negative Symptoms Scale (PANSS; P: Positive, N: Negative, G: General subscore segments marked by respective letters), the Schizophrenia Proneness Instrument - Adult (SPI-A) and the Structured Interview for Prodromal Syndromes (SIPS) before (red bars) and after (green bars) treatment. Significant clinical improvement was observed, reflected by the substantial reduction in scale scores. (B) FDG positron emission tomography scans of the brain before (pre) and after treatment (post) and the difference between post- and pre-treatment. (C) Increase in local cerebral glucose utilization after treatment in specific brain regions (in %). Brain regions (striatum, medial temporal cortex and midbrain) previously reported to be functionally modulated in CHR individuals by a single oral dose of 600 mg cannabidiol in a functional MRI study (11) are highlighted by red arrows.