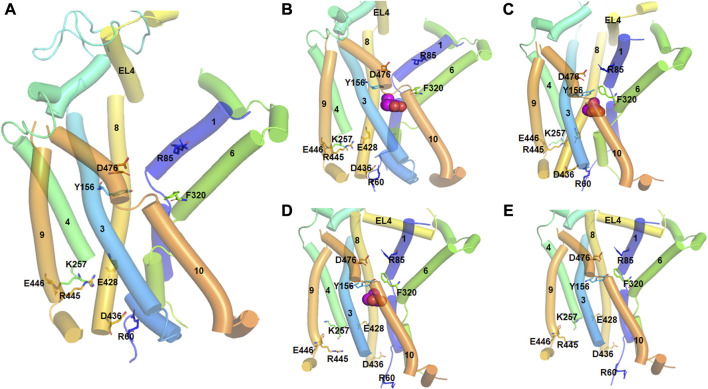
FIGURE 2.
Different conformational states of hDAT during the transport cycle. The TM helices are numbered and represented by tubes. Important residues involved in conformational changes are shown in stick model and labeled. Substrate molecule (DA) is represented in spherical model. (A). In the outward-open state (apo form), the salt bridge between D476 (TM10) and R85 (TM1) which acts as the first extracellular gate is interrupted. Similarly, the interaction between Y156 and F320 which function as the second extracellular gate is also broken. At the intracellular side, there are strong ionic interactions between E448-K257, R445-E428 and D436-R60 residues. Particularly, D436-R60 acts as the intracellular gate keeper. (B). In the outward-open substrate bound state, a DA molecule binds at the orthostatic binding site. All the ionic interactions are the same as in the apo form. (C). In the substrate-occluded state, the extracellular gates, D476-R85 and Y156 and F320 are closed. There is also movement of the EL4 loop to close the extracellular vestibule. (D). In the inward-open state, the ionic interactions E448-K257, R445-E428 are interrupted, and the intracellular gate formed by D436-R60 is opened. (E). In the inward-open state (apo from), DA is released into the intracellular sites and the cycle is reset.