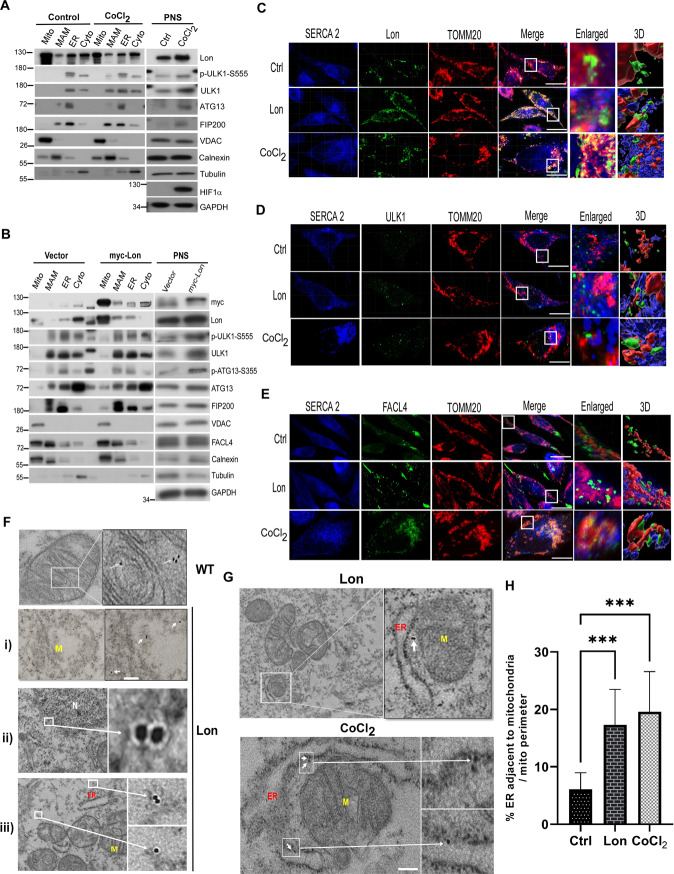
Fig. 4. Lon and ULK1 complex accumulates at ER-mitochondria tethering sites in response to hypoxia.
A, B Lon and ULK1 complex accumulates at ER-mitochondria contact (EMC) sites under hypoxia. HCT-15 cells treated with or without CoCl2 (200 μM for 18 h) (A) or transfected with the plasmids encoding myc-Lon (B) were used to perform subcellular fractionation experiment. Cell lysates were analyzed by immunoblotting using the indicated antibodies. GAPDH as the loading control. Mito mitochondria, MAM mitochondria associated membranes, ER endoplasmic reticulum, cyto cytosol, PNS post nuclear supernatant. C–E Accumulation of Lon and ULK1 at the EMC/MAM was verified by confocal immunofluorescence. HCT-15 cells were transfected with the plasmids encoding Lon or treated with CoCl2 (200 μM for 18 h) or not. The cells were fixed and immunostained by anti-Lon (green) (C), anti-ULK1 (green) (D), anti-FACL4 (ER and MAM, green) (E), anti-SERCA-2 (ER, blue), and anti-TOMM20 (mitochondria, red) antibodies. Scale bars = 10 μm. F–H Localizaton of Lon at the EMC/MAM was verified by transmission electron microscopy (TEM). HCT-15 cells were treated with CoCl2 (200 μM for 18 h). The cells were fixed and and immunostained by anti-Lon and immunogold labeling antibodies. The immunogold electron micrographs showed Lon in (i) damaged mitochondria (M), (ii) ER around Nucleus (N), (iii) Cytosol (F) and the ER-mitochondria tethering sites (G). ER: endoplasmic reticulum. Scale bars: 100 nm. Quantitation of the percentage of ER adjacent to mitochondria in both CoCl2 and Lon expressed HCT-15 cells and compared with control (H). The percentage was normalized by total ER and mitochondrial perimeter (n = 36 field for each condition). n = 3 biological replicates. The error bars shown in the panel represent the standard deviation from three independent experiments. ***p < 0.001.