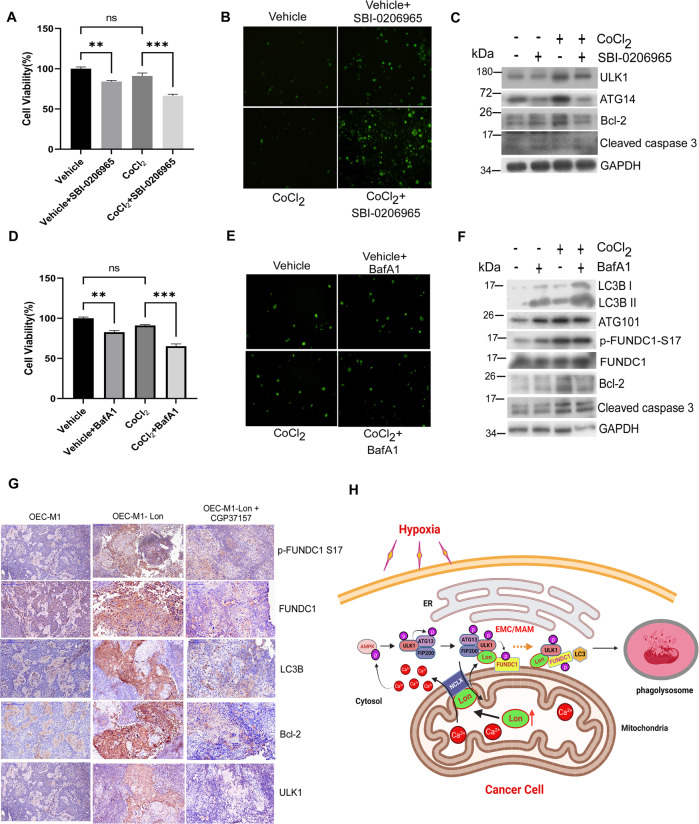
Fig. 8. Lon-ROS-ULK1-FUNDC1 axis induced mitophagy benefits cell survival and tumorigenesis in vitro and in vivo.
A–C HCT-15 cells were treated with CoCl2 (200 μM for 18 h) or not in the presence or absence of SBI-0206965 (20 μM for 24 h). The MTS assay for cell viability (A), fluorescence-based cleaved Caspase 3 apoptosis assay (B), and western blotting analysis (C) were performed. Immunoblots were obtained using the indicated antibodies. Scale bar, 100 μm (n = >50 cells/condition and 3 biological replicates). D–F HCT-15 cells were treated with CoCl2 (200 μM for 18 h) or not in the presence or absence of Bafilomycin A1 (100 nM for 24 h). The MTS assay for cell viability (D), fluorescence-based cleaved Caspase 3 apoptosis assay (E), and Western blotting analysis (F) were performed. Immunoblots were obtained using the indicated antibodies. Scale bar, 100 μm (n = >50 cells/condition and 3 biological replicates). G Immunohistochemical analysis of ULK1, p-FUNDC1 S17, FUNDC1, LC3B, and Bcl-2 expression in Lon expressed OEC-M1 tumor generated in BALB/C Nu mice treated with or without CGP37157. Representative immunohistochemical staining of respective targets was performed using paraffin-embedded sections of tumors collected. Microscopic magnification, ×400. Scale bar, 50 μm. H Scheme of the interaction between mitochondrial Lon and ULK1 complex at the EMC/MAM promotes mitophagy under hypoxia by stabilizing FUNDC1-ULK1 complex that depends on mitochondrial Na+/Ca2+ exchanger (NCLX). Upon hypoxia, Lon promotes FUNDC1-ULK1-mediated mitophagy at the EMC/MAM site, which is dependent on the binding with mitochondrial Na+/Ca2+ exchanger (NCLX). This interaction stabilized the FUNDC1-ULK1 at the EMC and initiated the mitophagy through the regulation of Ca2+ levels between mitochondria and cytosol. Lon-ULK1 phosphorylates FUNDC1 at S17, and Lon-ULK1-FUNDC1 axis promotes mitophagosome-lysosome fusion. EMC ER-mitochondria contact sites, MAM mitochondria associated membranes. The scheme of this study was created with BioRender.com.