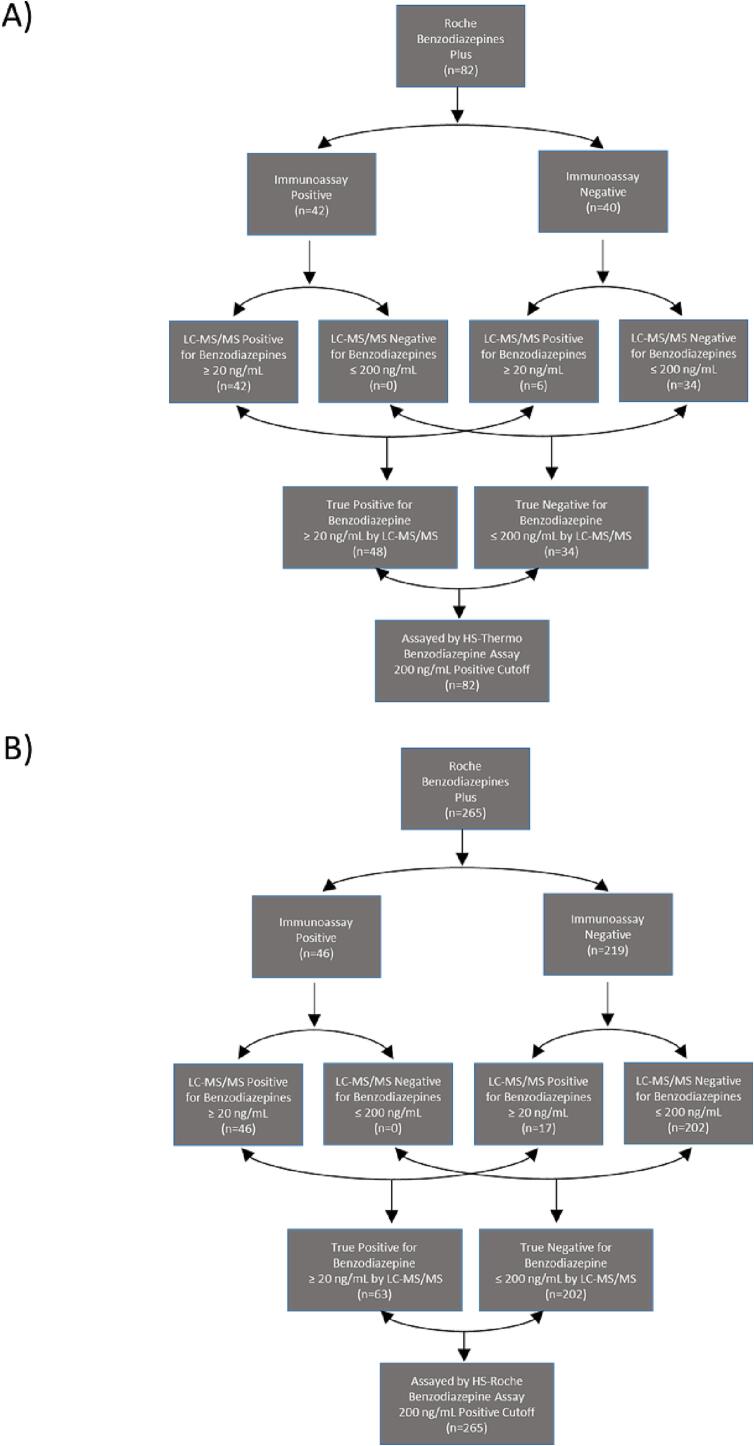
Fig. 1.
Classification of Clinical Specimens Used to Evaluate Commercial HS Benzodiazepine Immunoassays
Clinical urine specimens were screened during live testing using the Roche Benzodiazepine Plus immunoassay and categorized based on the screening result (positive vs. negative). LC-MS/MS analysis was used to stratify the samples based on the level of total benzodiazepines to determine the sensitivity and specificity (Y-axis) of the HS-Thermo (A) and HS-Roche (B) benzodiazepine immunoassays in clinical specimens. True positives and true negatives were classified based on the total concentration of benzodiazepines within a specimen as measured by LC-MS/MS (true positive ≥ 20 ng/mL; true negative ≤ 200 ng/mL).