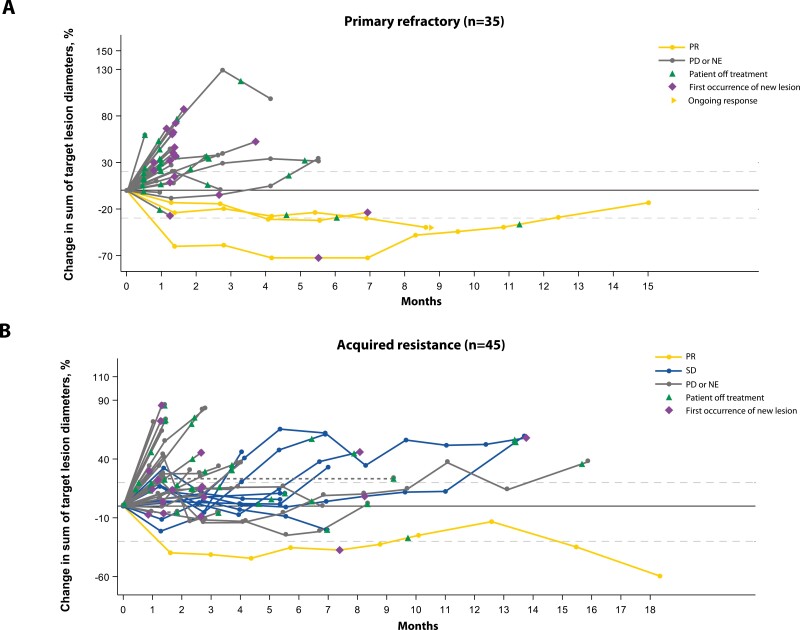
Figure 2.
Clinical activity of bintrafusp alfa. Percentage change in target lesion diameters over time as adjudicated by the IRC per RECIST 1.1 in patients with NSCLC that was primary refractory, ie, no disease control with prior anti-PD-(L)1 therapy (A) or had acquired resistance, ie, initial disease control with prior anti-PD-(L)1 therapy, followed by subsequent progression (B). Dashed lines at 20% and –30% indicate thresholds for progressive disease and partial response, respectively. Abbreviations: IRC, independent review committee; NE, not evaluable; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; RECIST 1.1, Response Evaluation Criteria in Solid Tumors version 1.1; SD, stable disease.