Figure 1.
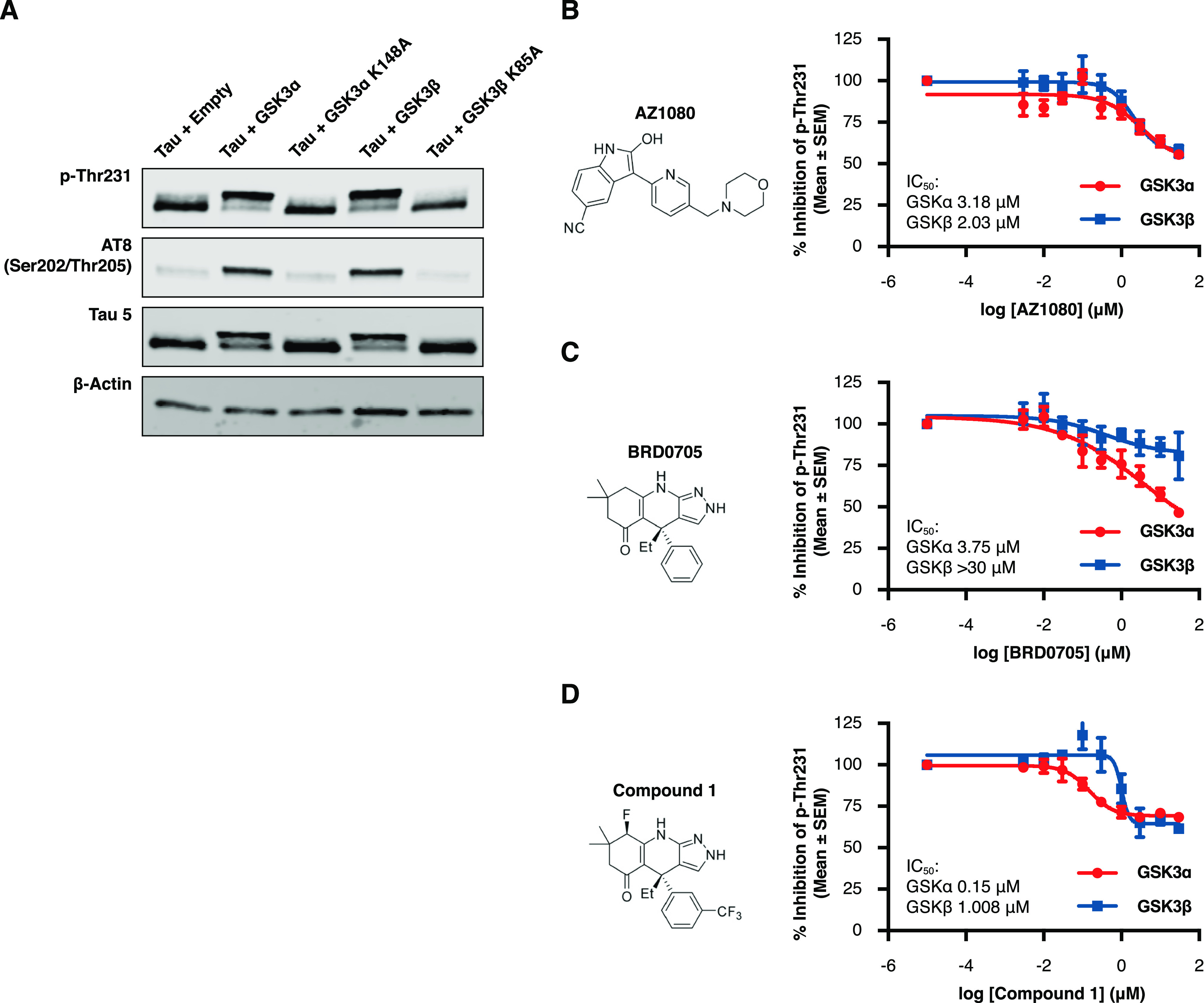
Acute and selective inhibition of GSK3α can reduce tau phosphorylation at disease relevant phospho-sites. HEK293 cells stably expressing human 2N4R tau (HEK-huTau) were transfected with plasmids expressing either human GSK3α, human GSK3β, or kinase dead mutants in which the catalytic lysine was mutated to alanine. 24 h after transfection, cells were lysed. (A) HEK-huTau lysates were run by SDS PAGE to probe for phosphorylation of tau at different epitopes. Both GSK3 isoforms phosphorylate tau at multiple epitopes including the disease enriched epitopes Thr231 and S202/Thr205 (AT8). (B–D) 24 h after transfection, compounds were added at a 10-point dose response (range: 3 nM–30 μM) for 2 h and cell lysates were probed for total tau (Tau5 antibody) and pThr231 using a plate-based assay. The figures represent IC50 curves demonstrating cellular potency and GSK3 isoform selectivity of the non-paralog-selective compound AZ1080 (B), a previously reported GSK3α selective compound BRD0705 (C), and a novel GSK3a selective small molecule, compound 1 (D). Each data point represents the mean ± the standard error of the mean (SEM) from four biological replicates within single run.
