Figure 4.
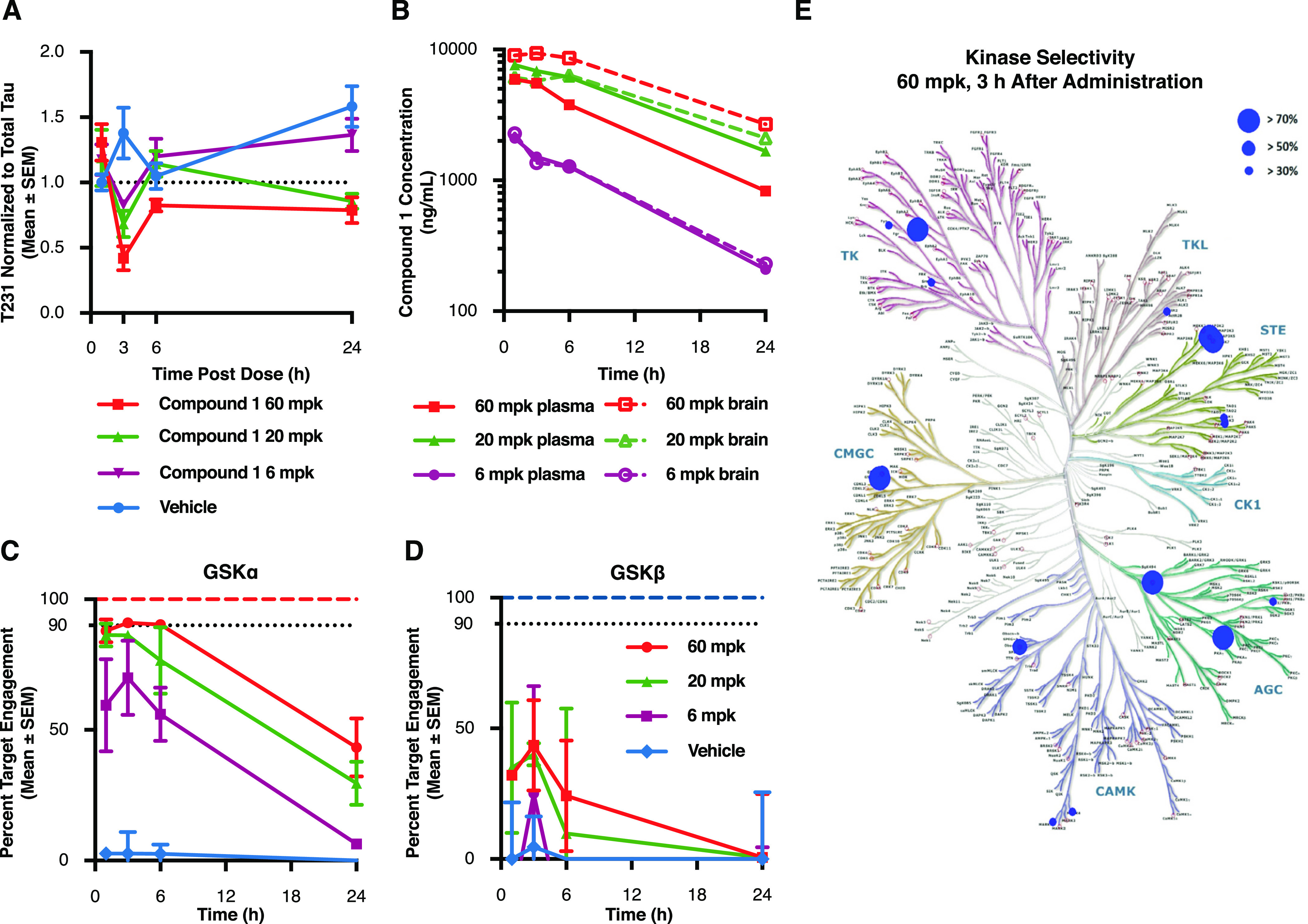
Acute GSK3α inhibition reduces tau phosphorylation at disease relevant sites in vivo. P10 rats were injected with compound 1 at 60, 20, and 6 mg/kg and sacrificed at several time points following treatment. (A) Cortical lysates were run in a plate-based assay to quantify levels of phosphorylated Thr231 normalized to total tau. Results demonstrate a significant lowering of T231 phosphorylation beginning 3 h after drug administration when compared to vehicle treated controls (60 mpk p = 0.004; 20 mpk p = 0.0227). Each data point represents the mean ± SEM from three animals. (B) To determine drug exposure, blood and cortical tissue were analyzed at each time point following dosing. Pharmacokinetic data demonstrate dose responsive compound concentrations with a steady Cmax extending to ∼6 h post injection at the highest dose and comparable exposure between blood and brain at each of the doses tested. (C,D) Target engagement at the GSK3 isoforms was measured using competitive chemoproteomics with Sepharose “kinobeads”. Results demonstrate that compound selectivity for the GSK3α paralog was retained in vivo. Each data point represents the mean ± SEM from three animals. (E) Competitive chemoproteomics was also used to monitor other kinases inhibited by compound 1. Our results show that at the highest dose tested (60 mg/kg), there were only 7 out of 251 unique kinases detected (including GSK3α) that had a target engagement of >70%.
