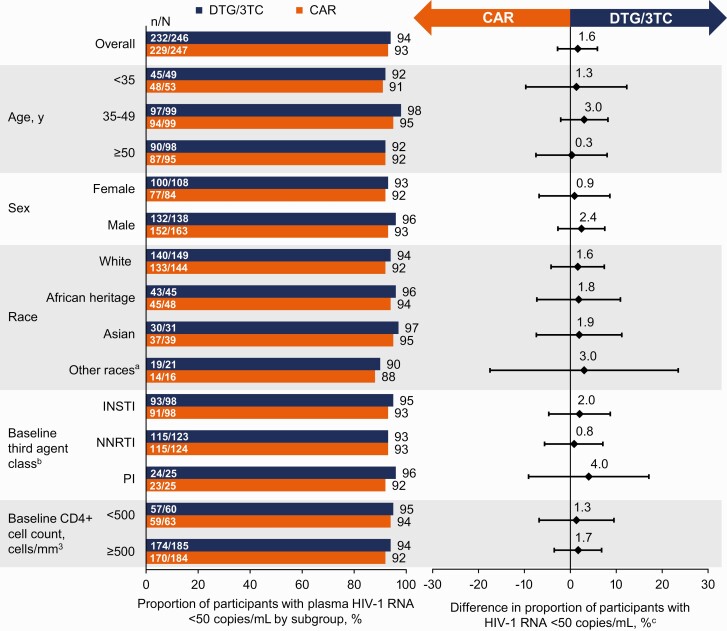
Figure 3.
Proportion of participants with HIV-1 RNA <50 copies/mL by subgroup in the ITT-E population by FDA Snapshot algorithm at week 48. aIncludes American Indian or Alaska Native or individuals of multiple races. bThe study population was stratified by baseline third agent class (PI, INSTI, or NNRTI). cAdjusted difference for overall population (DTG/3TC − CAR) and 95% CIs are based on a stratified analysis (adjusting for baseline third agent class) using Cochran-Mantel-Haenszel weights (meeting noninferiority based on −12% margin); unadjusted difference for subgroups calculated by proportion on DTG/3TC − proportion on CAR. Abbreviations: CAR, current antiretroviral regimen; CI, confidence interval; DTG/3TC, dolutegravir/lamivudine; FDA, Food and Drug Administration; HIV-1, human immunodeficiency virus 1; INSTI, integrase strand transfer inhibitor; ITT-E, intention-to-treat–exposed; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.