Figure 1:
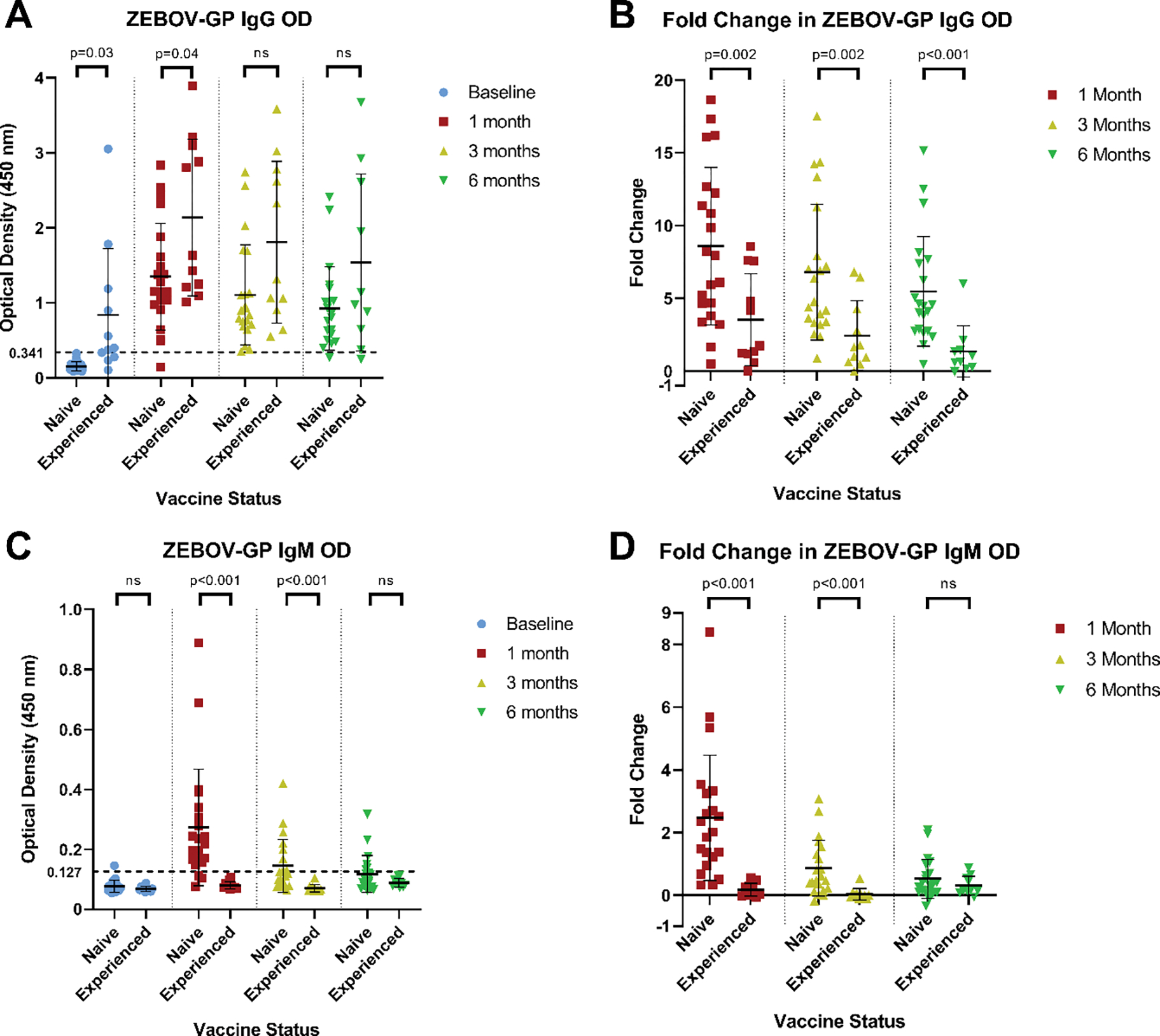
Antibody responses to ZEBOV-GP by ELISA
A: Comparison of ZEBOV-GP IgG ODs between ZEBOV vaccine naïve and ZEBOV vaccine experienced participants. B: Fold changes in ZEBOV-GP IgG ODs post-vaccination compared to baseline according to ZEBOV vaccination status at enrollment. C: Comparison of ZEBOV-GP IgM ODs between ZEBOV vaccine naïve and ZEBOV vaccine experienced participants. D: Fold changes in ZEBOV-GP IgM ODs post-vaccination compared to baseline according to ZEBOV vaccination status at enrollment. All ODs reflect a 1:100 serum dilution. Dotted line indicates the OD criterion used to define a positive antibody response to vaccination (at least three standard deviations above the mean OD at baseline value of ZEBOV vaccine naïve participants)
