Abstract
Introduction:
Clinical trial designs based on the assumption of independent observations are well established. Clustered clinical trial designs, where all observational units belong to a cluster and outcomes within clusters are expected to be correlated, have also received considerable attention. However, many clinical trials involve partially clustered data, where only some observational units belong to a cluster. Examples of such trials occur in neonatology, where participants include infants from both singleton and multiple births, and ophthalmology, where one or two eyes per participant may need treatment. Partial clustering can also arise in trials of group-based treatments (e.g. group education or counselling sessions) or treatments administered individually by a discrete number of health care professionals (e.g. surgeons or physical therapists), when this is compared to an unclustered control arm. Trials involving partially clustered data have received limited attention in the literature and the current lack of standardised terminology may be hampering the development and dissemination of methods for designing and analysing these trials.
Methods and examples:
In this article, we present an overarching definition of partially clustered trials, bringing together several existing trial designs including those for group-based treatments, clustering due to facilitator effects and the re-randomisation design. We define and describe four types of partially clustered trial designs, characterised by whether the clustering occurs pre-randomisation or post-randomisation and, in the case of pre-randomisation clustering, by the method of randomisation that is used for the clustered observations (individual randomisation, cluster randomisation or balanced randomisation within clusters). Real life examples are provided to highlight the occurrence of partially clustered trials across a variety of fields. To assess how partially clustered trials are currently reported, we review published reports of partially clustered trials.
Discussion:
Our findings demonstrate that the description of these trials is often incomplete and the terminology used to describe the trial designs is inconsistent, restricting the ability to identify these trials in the literature. By adopting the definitions and terminology presented in this article, the reporting of partially clustered trials can be substantially improved, and we present several recommendations for reporting these trial designs in practice. Greater awareness of partially clustered trials will facilitate more methodological research into their design and analysis, ultimately improving the quality of these trials.
Keywords: Clinical trials, trial design, clustering, partial clustering, partial nesting, terminology, reporting guidelines, re-randomisation, group-based treatment, individually randomised group treatment
Introduction
Statistical methods for clinical trials are frequently based on an assumption of independence, such that each participant contributes a single outcome and the outcome of one participant is unrelated to the outcome of another. The independence assumption is violated in trials that involve some form of clustering, such as when an intervention is delivered to groups of participants. When clustering is present, outcomes measured on participants from the same group or cluster are expected to be similar or correlated.1 This correlation may be due to existing similarities among individuals, for example, due to being located in the same geographical region or belonging to the same family. Alternatively, the correlation may be induced by the experimental design, for example, when otherwise independent individuals are allocated to receive treatment from the same clinician. Ignoring the clustering in such a trial can lead to it being over or under powered2 and incorrect type I error rates,3 and may therefore result in misleading conclusions.
There are many randomised trial designs that result in clustered data, broadly referred to hereafter as clustered trials. Common clustered trial designs are well described in the literature and include individually randomised cross-over trials (where multiple measurements are taken on each participant under different treatment conditions)4, parallel cluster randomised trials (where entire clusters are randomised, so that all participants within a cluster are allocated to the same treatment condition)5 and longitudinal variants such as stepped wedge trials and cluster randomised cross-over trials.6,7 Design and analysis approaches for clustered trials have been developed that account for the resulting non-independent data.7–12 Reporting guidelines have also been developed that provide a standardised framework for the description and reporting of such trials.13–17
While fully clustered trials where each individual or observation is included in a cluster have been extensively studied, limited attention has been given to partially clustered trials, where only some observations belong to a cluster. We define a partially clustered trial as any trial in which, by design, some observations are independent from all others, while other observations belong to a cluster. These trials occur across a range of health areas including mental health (where intervention arm participants receive treatment in group sessions, compared to an ungrouped control arm),18,19 neonatology (where participants include infants from both singleton and multiple births)20 and ophthalmology (where one or two eyes per participant may need treatment).21,22 Allowing inclusion of both independent and clustered observations can be advantageous. By widening the eligible population, a partially clustered trial may be more generalisable and result in faster recruitment, shorter trial duration and lower costs than other trial designs. Under certain scenarios, partially clustered trials can also have greater statistical power than a fully independent design.23
Although partially clustered trials arise in many settings, the lack of standardised terminology to refer to these trials may be hampering the development and dissemination of methods for their design and analysis. The aims of this article are to highlight the occurrence of partially clustered trials in practice, describe the different types of partially clustered trial designs and propose a standardised approach for describing these trials in the scientific literature. Our overall goal is to bring together the somewhat disparate research that has been presented for specific types of partially clustered trials or in specific health fields under the general umbrella of partially clustered trials.
Methods and examples
Partially clustered trials: a broad definition
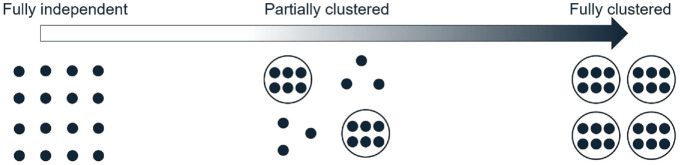
We define an observational unit as the unit on which the primary outcome is measured, contributing one observation, and a cluster as a group of multiple related observational units. In some settings, people are observational units and clusters consist of multiple people (e.g. clusters of patients who are treated by the same physician). In other settings, people are clusters and contribute multiple observations (e.g. outcomes measured on two eyes from the same person). Fully independent data are defined as each observation being independent from all others, and fully clustered data as each observation belonging to a cluster of size greater than or equal to two, where outcomes of observational units from the same cluster are correlated. Partially clustered data are then defined as a mixture of independent and clustered observations, which may fall anywhere on a clustering continuum from fully independent data at one extreme and fully clustered data at the other extreme (Figure 1).
Figure 1.
The clustering continuum from fully independent to fully clustered data. Each dot represents an observation. Dots grouped together in a circle represent a cluster of correlated observations. Ungrouped dots represent the independent observations.
Following on from these definitions, we broadly define a partially clustered trial as any trial that is designed to include at least one independent observation and one cluster of observations (Table 1). Our definition of partially clustered trials does not include trials that are intended to be fully clustered but end up including some independent observations due to missing data or intermediate events occurring to other cluster members. We restrict attention to designs with only one level of clustering and a single randomisation of observational units or clusters to treatments, since multiple levels of clustering or randomisation introduce additional complexities that are beyond the scope of this article. However, we recognise that such designs may be affected by partial clustering at one or more levels.24,25 Under our definition, partially clustered trials can be seen as an umbrella term used to encompass a wide range of specific designs, as described below.
Table 1.
A summary of terms.
| Term | Definition |
|---|---|
| Observational unit | Unit on which the primary outcome is measured |
| Cluster | A collection of multiple related observational units |
| Observation level/observational unit level | Interventions applied to, or measurements taken on, individual observational units |
| Cluster level | Interventions applied to, or measurements taken on, clusters as a whole |
| Fully independent trial | A randomised trial where each observation is independent of all others |
| Partially clustered trial | A randomised trial that includes, by design, both independent observations and clustered observations |
| (a) Individual randomisation with post-randomisation clustering | A type of partially clustered trial where observational units are independently randomised to treatment arms, clusters are formed after randomisation, and observations are fully (or partially) clustered in some treatment arms and are fully independent in other treatment arms |
| (b) Individual randomisation within pre-existing clusters | A type of partially clustered trial where clusters exist prior to randomisation and observational units within clusters are independently randomised to treatment arms |
| (c) Cluster randomisation of pre-existing clusters | A type of partially clustered trial where clusters that exist before randomisation are allocated to treatment arms |
| (d) Balanced randomisation within pre-existing clusters | A type of partially clustered trial with balanced allocation of observational units to treatments within clusters that exist prior to randomisation |
| Fully clustered trial | A randomised trial where each observation belongs to a cluster of size greater than or equal to two |
Types of partially clustered trial designs
The specific type of partially clustered trial is determined by two factors. First, the clusters may exist before randomisation (e.g. body parts of a person) or may be induced only after participants have been randomised (e.g. by assigning people in the same treatment arm to group-based education classes). In fully clustered trials, this has been referred to as pre-randomisation and post-randomisation clustering, respectively, and we adopt these terms throughout,26 although it is sometimes referred to as ‘recruitment-related’ and ‘treatment-related’ clustering,27 or ‘natural’ and ‘imposed’ clustering, respectively.28 Second, the method of randomisation for the clustered observations can vary when clusters exist prior to randomisation. For example, two eyes from the same person may be randomised independently (using individual randomisation of each observational unit), assigned to the same treatment arm (using cluster randomisation) or placed in different treatment arms (using balanced randomisation of observational units within clusters). Pre-randomisation clusters are typically small in many fields (e.g. two eyes in ophthalmology), but can be much larger (e.g. up to 19 warts per patient in a dermatology trial29).
These two factors lead to four types of partially clustered designs: one design where clusters do not exist before randomisation and hence randomisation must be performed at the observational unit level, and three designs for clusters that exist before randomisation that differ based on the randomisation method used within clusters. These four designs are described in detail below and summarised in Table 2. Additional examples of each design type are provided in the Supplemental Material (Appendix 1).
Table 2.
Design features of different kinds of partially clustered trials.
| Trial type | Figure reference | Cluster membership exists before randomisation | Level of randomisation | Clustering in all treatment arms | Level of intervention | Alternative names/sub-types of trial designs |
|---|---|---|---|---|---|---|
| Individual randomisation with post-randomisation clustering | 2(a) | No | Individual randomisation of observational units | No | Cluster or observational unit | Individually randomised group treatment
design; Group-based treatment design; Facilitator/therapist effect design; Partially nested therapist design; Partially crossed therapist design |
| Individual randomisation within pre-existing clusters | 2(b) | Yes | Individual randomisation of observational units | Yes | Observational unit | Re-randomisation design |
| Cluster randomisation of pre-existing clusters | 2(c) | Yes | Cluster randomisation | Yes | Cluster or observational unit | – |
| Balanced randomisation within pre-existing clusters | 2(d) | Yes | Restricted individual randomisation of observational units | Yes | Observational unit | – |
Individual randomisation with post-randomisation clustering
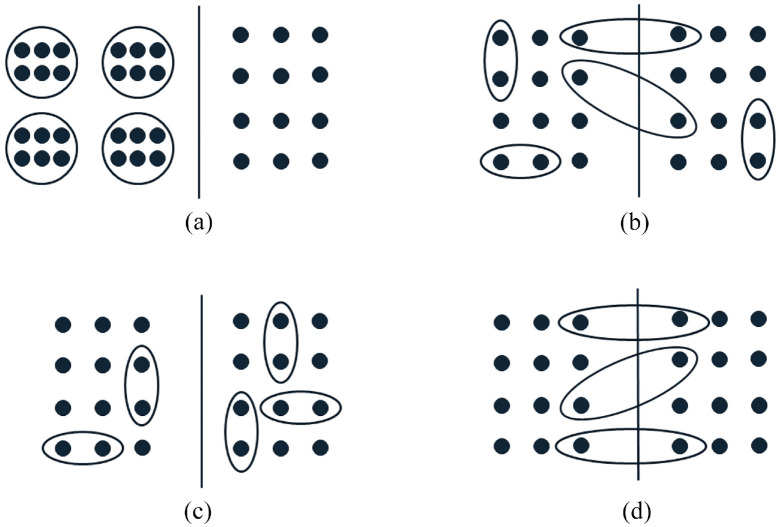
Partial clustering can occur in a trial when cluster membership is established after individual randomisation (i.e. independent randomisation of each observational unit) to treatment arms, and clustering occurs in some, but not all, treatment arms (Figure 2(a)). In this design, clustering is induced by the implementation of the intervention, and we refer to this design as a partially clustered trial using individual randomisation with post-randomisation clustering. An example of this design is the widely discussed group-based treatment design for the assessment of interventions administered at the cluster level, such as education classes, group counselling sessions or group physical activity sessions, compared to an unclustered control arm.30,31 The intervention arm consists of fully clustered observations, while observations in the control arm are fully independent. Post-randomisation clustering can also arise from the interventions that are administered individually, but by a discrete number of health practitioners such as surgeons, counsellors and physical therapists.32,33 This clustering has been termed the ‘therapist effect’ or ‘facilitator effect’30 and typically results in fully clustered observations nested within the intervention arm, although partial clustering within this arm is possible if some facilitators administer treatment to only one participant, and designs where clusters are crossed with treatment are also possible.24 The term ‘individually randomised group treatment’ design was also proposed for trials of group-based treatments, including both fully and partially clustered designs,34 although it is now used more widely for both group-based treatment and facilitator effect designs.35 Within some fields, the term ‘partial clustering’ or ‘partial nesting’ is used to exclusively refer to trials with individual randomisation and post-randomisation partial clustering;24,36 however, we consider this design to be just one of four possible partially clustered trial designs that fall under our broader definition.
Figure 2.
Graphical representations of different types of partially clustered trials. Each panel represents a two-arm trial with the left and right sides of the solid line depicting the two treatment arms. Each dot represents an observation. Dots grouped together in a circle/ellipse represent a cluster of correlated observations. Ungrouped dots represent the independent observations. (a) Individual randomisation with post-randomisation clustering. (b) Individual randomisation within pre-existing clusters. (c) Cluster randomisation of pre-existing clusters. (d) Balanced randomisation within pre-existing clusters.
Example trial
The Scleroderma Patient-centred Intervention Network COVID-19 Home-isolation Activities Together (SPIN-CHAT) Program is a support programme for people with an existing medical condition during COVID-19. The impact of the programme on anxiety symptoms after the 4-week intervention period was assessed in a partially clustered trial.18 Eligible participants were individually randomised to the intervention or a waitlist control arm. The intervention was delivered virtually to clusters of participants, whereas the waitlist control arm received no contact during the intervention period resulting in a group-based treatment design. The trial randomly assigned 172 participants to intervention or control (n = 86 in each arm), and intervention participants were allocated to 1 of 11 clusters of 6–10 participants for delivery of the intervention.
Individual randomisation within pre-existing clusters
If clusters exist before randomisation (i.e. pre-randomisation clustering) and observational units are independently assigned to treatment arms using individual randomisation, all members within some clusters may be randomised to the same treatment arm, while other clusters may be split across treatment arms (Figure 2(b)). We refer to this design as a partially clustered trial with individual randomisation within pre-existing clusters. Since observational units within the same cluster may be allocated to different treatment arms, the intervention must be administered at the observational unit level. Trials utilising individual randomisation of pre-existing clusters have been used in various fields, including neonatal health,37 antenatal health,38 fertility treatment39 and chronic liver failure.40 The re-randomisation design is a specific example of this type of design, where participants may enrol into the trial on multiple occasions and are randomised independently at each enrolment.41 Re-randomisation designs can increase the recruitment rate and decrease time to trial completion.42
Example trial
The Omega-3 (N-3) Fatty Acids for Improvement in Respiratory Outcomes (N3RO) trial was a randomised controlled trial involving preterm infants who were randomised to either an omega-3 or control emulsion that was administered enterally.37 The primary outcome was the incidence of physiological bronchopulmonary dysplasia measured at 36 weeks postmenstrual age or discharge home, whichever occurred first. Infants from single or multiple births were eligible for the trial and infants from the same birth were randomised independently, resulting in a partially clustered design with individual randomisation within pre-existing clusters (families). A total of 1273 infants from 1098 families were included in the trial.
Cluster randomisation of pre-existing clusters
If cluster-level randomisation is used with pre-existing, pre-randomisation, clusters, the result is a partially clustered trial where clusters may occur in all treatment arms and all members of the same cluster will be allocated to the same treatment arm. Non-clustered observational units are randomised independently, leading to each treatment arm containing a mix of independent and clustered observations (Figure 2(c)). We refer to this design as a partially clustered trial with cluster randomisation of pre-existing clusters. Under this design, the intervention may be administered at either the cluster level or observational unit level. Cluster randomisation is the only choice for treatments administered at the cluster level (e.g. a systemic medication that affects both eyes in an ophthalmology trial). Partially clustered trials with cluster randomisation of pre-existing clusters have been reported in fields including pregnancy and neonatal health,43–49 sports medicine,50 ophthalmology51 and surgery.52
Example trial
A surgical trial compared two mesh prosthetics placed during minimally invasive inguinal hernia repair.52 Patients were randomised to surgery with either a flat lightweight polypropylene mesh or a preformed lightweight mesh. Patients with both unilateral and bilateral inguinal hernias were eligible, and patients with bilateral hernias received the same mesh treatment for both procedures, resulting in a partially clustered trial with cluster randomisation of pre-existing clusters (patients). The primary outcome was the time for the mesh placement during surgery, measured on each hernia. Forty-eight patients were enrolled for a total of 65 hernia repairs.
Balanced randomisation within pre-existing clusters
When clusters exist pre-randomisation, a trial may be designed to balance treatment allocations across observational units within clusters. For example, if clusters have a maximum size of two and there are two treatment arms, one cluster member is randomised to a treatment arm and the other cluster member is then automatically assigned to the other treatment arm. Each cluster therefore receives both treatments (Figure 2(d)) as occurs in matched-pair designs (e.g. crossover trials) in the fully clustered setting. We refer to this design as a partially clustered trial with balanced randomisation within pre-existing clusters. The intervention must be administered at the observational unit level under this design. Balanced randomisation is most commonly seen in settings involving paired body parts (e.g. eyes, joints) where clusters naturally have a maximum size of two,53–56 although it can also be used with larger cluster sizes or more than two treatment arms.57
Example trial
An ophthalmology trial evaluated the effectiveness of ciliary neurotrophic factor (CNTF) for the treatment of macular telangiectasia type 2, a degenerative disease of the retina.53 Participants were eligible if one or both eyes met the inclusion criteria, leading to a partially clustered trial. The intervention was administered via a surgical implant, or a sham surgery if in the control group, into the study eye. The primary outcome was the change in the area of the ellipsoid zone disruption of the study eye at 24 months. For participants with both eyes eligible, the right eye was randomised to CNTF or sham surgery, and the left eye was allocated to the alternate treatment, resulting in balanced treatment allocations within clusters. A total of 99 eyes from 67 participants (clusters) were randomised into the study.
Choice of partially clustered trial design
Some characteristics of a partially clustered trial will be determined by the population under study, the type of intervention, and logistical issues around the recruitment of participants and the delivery of the intervention. If the intervention is delivered in groups formed after randomisation or by one of several facilitators and an ungrouped comparator arm is to be used, then researchers will be implementing a partially clustered trial using individual randomisation with post-randomisation clustering. When clusters exist before randomisation, researchers may be able to choose the randomisation method for the clustered observations (cluster, individual or balanced), and this will dictate which partially clustered trial design is used. Individual randomisation can be used when the treatment is assigned at the observational unit level, and there is minimal risk of contamination of the treatment between cluster members. Cluster randomisation may be preferred due to logistical reasons,8 when the treatment of one cluster member has the potential to influence other cluster members,58 or participant preferences, especially when clusters are twins.59 Balanced randomisation within clusters may have ethical advantages in trials where clusters are participants, as it ensures each participant will be exposed to the novel or gold standard treatment,60 and can result in faster recruitment and lower trial costs.61 The method of randomisation within pre-existing clusters can impact the statistical power and target sample size of a trial, with balanced randomisation providing the greatest power.23 The feasibility, pros and cons of using each method of randomisation for pre-existing clusters should be carefully considered in the context of the individual trial before determining which type of partially clustered design to use.
Problems identifying partially clustered trials
There are currently no reporting guidelines specifically for partially clustered trials. With no consistent terminology in place, it is difficult to identify reports of partially clustered trials and hence conduct a systematic review of current reporting practices to inform recommendations for describing partially clustered trial designs. Instead, we identified a convenience sample of protocols and reports of partially clustered trials and examined how the designs were reported to provide some initial insight. Reports were identified from citations of the small number of existing methodological papers on partially clustered trials, a literature search for ‘partial clustering’ or ‘partial nesting’, and by ad hoc inspection of recent issues of clinical trial and medical journals. Given the way these trials were identified, they are not expected to be representative of all published partially clustered trials and may overestimate the quality of reporting, as they likely represent some of the more easily identified partially clustered trials.
Our convenience sample included 40 articles (10 protocols and 30 results papers) covering all four types of partially clustered trials across a range of research areas, including education, psychology and medicine (Table 3). While most trials utilising individual randomisation with post-randomisation partial clustering used the terms ‘partially clustered’ or ‘partially nested’ within the publication (7/11), these terms were not used to describe trials of any other design type. Eleven re-randomisation trials were identified, only one of which was described as using a ‘re-randomisation design’.39 The use of re-randomisation in the other examples could only be inferred from the description of the design,62 patient population,63 eligibility criteria,64 or the randomisation process65 or results.66 For the other 18 partially clustered trials involving randomisation of pre-existing clusters but not using a re-randomisation design, the presence of clustering was not included in the primary description of the study design, even when other important design features were explicitly included (e.g. multicentre, double-blind, randomised controlled trial). Importantly, there was no consistency on where or how the partial clustering was described. Instead, the relevant information was variously included in the description of the study design,57 inclusion criteria,45 or the randomisation43,53 or treatment allocation56 processes. Overall, these findings suggest that current practices for describing partially clustered trial designs are inadequate.
Table 3.
Reporting practices in reports of 40 partially clustered trials.
| Convenience sample of partially clustered trials | Number of reports |
|---|---|
| Trial type | |
| Individual randomisation with post-randomisation clustering | 11 |
| Group-based treatment designs | 9 |
| Facilitator effect designs | 2 |
| Individual randomisation within pre-existing clusters | 13 |
| Re-randomisation designs | 11 |
| Other designs | 2 |
| Cluster randomisation of pre-existing clusters | 11 |
| Balanced randomisation within pre-existing clusters | 5 |
| Report type a | |
| Protocol paper | 10 |
| Primary results | 30 |
| Trial design reporting practices | |
| ‘Partial clustering’ or ‘partial nesting’ used in the main description of the trial design | 7 |
| Partial clustering inferred from the Methods section | 32 |
| Design sub-section | 3 |
| Participants/inclusion criteria sub-section | 3 |
| Intervention sub-section | 7 |
| Randomisation sub-section | 8 |
| Otherb | 11 |
| Partial clustering inferred from the results | 1 |
Protocol papers were included if a publication of the primary trial results could not be identified. The list of included reports is included in the Supplemental Material (Appendix 2).
‘Other’ includes combined sub-sections such as ‘participants and allocation’ and Methods sections without any sub-sections.
Recommendations for describing partially clustered trial designs
To facilitate the identification of partially clustered trials in the literature, clear reporting of the trial design is required. Reporting guidelines acknowledge the importance of clearly identifying the specific trial design in reports of protocols,67 trial results13–17,68,69 and in abstracts.70 We therefore recommend that the type of partially clustered trial should be described in reports of such trials using the terminology and design types described in this article (or other established terms, where applicable). While current guidelines recommend identifying the trial design in the title, the length of the partially clustered trial descriptions and the limited number of clusters in some settings suggest that it may be more useful to: (a) describe a partially clustered trial as a ‘randomised trial’ in the title, (b) describe the trial as ‘partially clustered’ or involving ‘partial clustering’, in both the abstract and methods and (c) describe the specific type of partially clustered design clearly in the methods, such that the method of randomisation and what constitutes the cluster-level and observation-level units are clear. Addressing this final recommendation may involve using the terminology for the trial designs introduced in this article, established terms for specific designs (such as re-randomisation or individually randomised group treatment designs) or language appropriate for the context of the particular trial. Examples of adequately described design descriptions for each type of partially clustered trial are provided in Table 4. Such detail provides transparency and context for interpreting trial details such as the level of treatment administration, the method of randomisation, sample sizes at the observation and cluster level and the statistical analysis methods.
Table 4.
Examples of adequately detailed descriptions of partially clustered trial designs for published trial reports.
| Trial type |
|---|
| Individual randomisation with post-randomisation clustering |
| ‘The trial was a partially clustered, individually
randomised group treatment trial with a waitlist control
group. Participants were randomised to intervention or
control, and within the intervention group facilitators
administered a health promotion program to small groups of
8–12 participants’. Group-based treatment design: ‘The trial was a two-arm parallel, partially clustered, randomised, controlled trial. Participants were individually randomised to intervention or control, and intervention participants were subsequently clustered into groups for delivery of the counselling sessions in a group-based treatment design’. Facilitator effect design: ‘The trial is designed as a multi-centre, parallel group, individually randomised, controlled trial with partial clustering. Physiotherapists will deliver the intervention to participants individually, with each physiotherapist expected to treat an average of 7 participants’. |
| Individual randomisation within pre-existing clusters |
| ‘The trial was a randomised, blinded, controlled trial, with
partial clustering of infants from multiple births. All
infants were randomised
individually’. Re-randomisation design: ‘The trial is a pragmatic, partially clustered, two-arm parallel-group randomised controlled trial using re-randomisation’. |
| Cluster randomisation of pre-existing clusters |
| ‘The trial is a prospective, multi-centre, open-label, randomised trial. If inclusion criteria were met, one or both eyes of eligible participants were enrolled in the study and received the same treatment, hence this a partially clustered trial with cluster randomisation’. |
| Balanced randomisation within pre-existing clusters |
| ‘This was a phase 3, single centre, randomised, partially clustered, controlled trial involving patients scheduled for elective vascular surgery with either unilateral or bilateral inguinal incisions. In the case of bilateral incisions, the right incision was randomised to intervention or control, and the left incision received the alternate treatment’. |
Source: Adapted from real examples of partially clustered trial reports.
Discussion
We have provided a unifying, broad definition of partially clustered trials and proposed standardised terminology for describing different partially clustered trial designs. Currently, there is a lack of consistent terminology used to describe partially clustered trials, which makes it difficult to systematically search the literature to identify published examples of such trials. Therefore, little is known about how they are designed, described and analysed in practice, including whether the clustering has been appropriately accounted for. Previous reviews on the use of partially clustered trials have been conducted within individual fields by searching complete issues of specialist journals.21,71,72 A similar review could be conducted in general medical journals, potentially identifying other applied fields where these trials are used, and this is a suggested area for future research. While the framework presented here is restricted to simple designs that involve a single level of clustering and one stage of randomisation, more complex designs involving partial clustering (including partial crossing of treatments and facilitators,24 and multitier designs that include more than one stage of random allocation24,25) are possible and our work could be extended to these scenarios in the future.
Limited research has been conducted to date on the design and analysis of partially clustered trials. Most methodological literature relates to partially clustered trials using individual randomisation with post-randomisation clustering.30,36,73 The statistical issues that arise in partially clustered trials involving pre-existing clusters have been discussed in specialty fields including ophthalmology,21 orthopaedics71,72 and surgery,74 but without the use of a specific term for this design. Recent methodological work has been conducted on sample size23 and multiple imputation75 for trials involving a combination of independent and paired data, but without reference to partial clustering. Sample size methods exist for some types of partially clustered trials.23,76,77 Research has been conducted on the analysis of partially clustered trials that arise in neonatology due to multiple births78–81 and of re-randomisation designs;41,82 however, reviews in specific subject-matter areas have found that many partially clustered trials are analysed using potentially inappropriate methods,71,72,83,84 suggesting that the importance of clustering in these designs is under-recognised. Appropriate analysis of partially clustered data is important to ensure correct estimates of variability and type I error rates,3 and partial clustering should be accounted for when determining sample size to avoid trials being over or under powered.2 Further development of sample size methods, assessment of appropriate analysis methods and analysis recommendations that are accessible to applied researchers are needed for the full range of partially clustered trial designs. While each design could be considered in isolation, we believe there is benefit in considering similarities across designs and how methods developed for one partially clustered trial design could be applied to or modified to suit other designs. Such development should be conducted with consideration of the estimand framework to ensure an appropriate definition of the desired treatment effect.85 Estimands have recently been defined for fully clustered designs86–88 and re-randomisation designs,89 and extensions to partially clustered designs more generally are a logical next step. We encourage researchers to follow the recommendations provided in this article when referring to different partially clustered designs in future methodological work to aid the dissemination of relevant methodological advances across applied areas.
Poor and inconsistent reporting of trial results, including unclear randomisation methods, is associated with bias in reported treatment effects.90,91 This is of particular relevance to partially clustered trials, where there are multiple methods available for randomisation of observational units within pre-existing clusters, and this may not always be clearly described. Critically assessing the quality and validity of trial results requires complete reporting of the trial design and implementation. Development of detailed reporting guidelines for partially clustered trials is thus an important area for future research. In the absence of specific guidelines, we encourage researchers and journals to refer to the relevant recommendations for fully clustered trials,13 non-pharmacologic treatments16,17 (in the case of individual randomisation with post-randomisation clustering) and within-person trials69 (in the case of balanced randomisation within pre-existing clusters), and the specific advice for partially clustered trials that we have presented in this article. However, following all recommendations for fully clustered trials may be challenging for some partially clustered trials, such as reporting an intraclass correlation coefficient for outcomes collected in partially clustered trials with few clusters, and hence some reporting discretion may be necessary based on the specific trial.
In conclusion, partially clustered trials occur across a range of applied areas. The lack of a unified definition of partially clustered trials and consistent terminology for describing the various types of designs limits the ability of researchers to accurately assess their use and develop methods for their design and analysis. By adopting standard definitions and terminology, such as those presented in this article, the reporting of partially clustered trials can be substantially improved. Greater awareness of these trials will facilitate more methodological research into their design and analysis for the benefit of clinical researchers.
Supplemental Material
Supplemental material, sj-docx-1-ctj-10.1177_17407745221146987 for Partially clustered designs for clinical trials: Unifying existing designs using consistent terminology by Kylie M Lange, Jessica Kasza, Thomas R Sullivan and Lisa N Yelland in Clinical Trials
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: K.M.L. is supported through an Australian Government Training Programme (RTP) Scholarship administered by The University of Adelaide, Australia, an Australian Trials Methodology (AusTriM) Research Network supplementary scholarship and a Statistical Society of Australia PhD top-up award. T.R.S. is supported by a National Health and Medical Research Council (NHMRC) emerging leadership fellowship APP1173576. This research was supported by a Centre of Research Excellence grant from the NHMRC, ID #1171422, to the AusTriM Research Network.
ORCID iDs: Kylie M Lange  https://orcid.org/0000-0003-3814-8513
https://orcid.org/0000-0003-3814-8513
Jessica Kasza  https://orcid.org/0000-0002-8940-0136
https://orcid.org/0000-0002-8940-0136
Supplemental material: Supplemental material for this article is available online.
References
- 1. Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann Fam Med 2004; 2(3): 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vierron E, Giraudeau B. Design effect in multicenter studies: gain or loss of power? BMC Med Res Methodol 2009; 9: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London: Arnold, 2000. [Google Scholar]
- 4. Senn SS. Cross-over trials in clinical research. 2nd ed. Chichester: Wiley, 2002. [Google Scholar]
- 5. Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health 2004; 94(3): 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015; 350: h391. [DOI] [PubMed] [Google Scholar]
- 7. Hemming K, Kasza J, Hooper R, et al. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. Int J Epidemiol 2020; 49: 979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006; 35(5): 1292–1300. [DOI] [PubMed] [Google Scholar]
- 9. Hooper R, Teerenstra S, de Hoop E, et al. Sample size calculation for stepped wedge and other longitudinal cluster randomised trials. Stat Med 2016; 35: 4718–4728. [DOI] [PubMed] [Google Scholar]
- 10. Campbell MK, Mollison J, Steen N, et al. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract 2000; 17(2): 192–196. [DOI] [PubMed] [Google Scholar]
- 11. Heo M, Leon AC. Comparison of statistical methods for analysis of clustered binary observations. Stat Med 2005; 24: 911–923. [DOI] [PubMed] [Google Scholar]
- 12. Hemming K, Taljaard M, Forbes A. Analysis of cluster randomised stepped wedge trials with repeated cross-sectional samples. Trials 2017; 18: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012; 345: e5661. [DOI] [PubMed] [Google Scholar]
- 14. Hemming K, Taljaard M, McKenzie JE, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ 2018; 363: k1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dwan K, Li T, Altman DG, et al. CONSORT 2010 statement: extension to randomised crossover trials. BMJ 2019; 366: l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008; 148: 295–309. [DOI] [PubMed] [Google Scholar]
- 17. Boutron I, Altman DG, Moher D, et al. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017; 167: 40–47. [DOI] [PubMed] [Google Scholar]
- 18. Thombs BD, Kwakkenbos L, Levis B, et al. Effects of a multi-faceted education and support programme on anxiety symptoms among people with systemic sclerosis and anxiety during COVID-19 (SPIN-CHAT): a two-arm parallel, partially nested, randomised, controlled trial. Lancet Rheumatol 2021; 3(6): e427–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janzarik G, Wollschläger D, Wessa M, et al. A group intervention to promote resilience in nursing professionals: a randomised controlled trial. Int J Environ Res Public Health 2022; 19: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yelland LN, Sullivan TR, Makrides M. Accounting for multiple births in randomised trials: a systematic review. Arch Dis Child Fetal Neonatal Ed 2015; 100(2): F116–F120. [DOI] [PubMed] [Google Scholar]
- 21. Karakosta A, Vassilaki M, Plainis S, et al. Choice of analytic approach for eye-specific outcomes: one eye or two? Am J Ophthalmol 2012; 153: 571.e1–579.e1. [DOI] [PubMed] [Google Scholar]
- 22. Lee CF, Cheng ACO, Fong DYT. Eyes or subjects: are ophthalmic randomized controlled trials properly designed and analyzed? Ophthalmology 2012; 119: 869–872. [DOI] [PubMed] [Google Scholar]
- 23. Yelland LN, Sullivan TR, Price DJ, et al. Sample size calculations for randomised trials including both independent and paired data. Stat Med 2017; 36: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 24. Walwyn R, Roberts C. Therapist variation within randomised trials of psychotherapy: implications for precision, internal and external validity. Stat Methods Med Res 2010; 19(3): 291–315. [DOI] [PubMed] [Google Scholar]
- 25. Brien CJ, Bailey RA. Multiple randomizations. J R Stat Soc Ser B Stat Methodol 2006; 68: 571–609. [Google Scholar]
- 26. Kahan BC, Morris TP. Assessing potential sources of clustering in individually randomised trials. BMC Med Res Methodol 2013; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walwyn R, Roberts C. Meta-analysis of absolute mean differences from randomised trials with treatment-related clustering associated with care providers. Stat Med 2015; 34: 966–983. [DOI] [PubMed] [Google Scholar]
- 28. Lee KJ, Thompson SG. Clustering by health professional in individually randomised trials. BMJ 2005; 330: 142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stender IM, Na R, Fogh H, et al. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial. Lancet 2000; 355: 963–966. [DOI] [PubMed] [Google Scholar]
- 30. Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005; 2(2): 152–162. [DOI] [PubMed] [Google Scholar]
- 31. Lohr S, Schochet PZ, Sanders E. Partially nested randomized controlled trials in education research: a guide to design and analysis. NCER 2014-2000, National Center for Education Research, https://eric.ed.gov/?id=ED545532 (July 2014, accessed 26 July 2021). [Google Scholar]
- 32. Sohanpal R, Pinnock H, Steed L, et al. Tailored, psychological intervention for anxiety or depression in people with chronic obstructive pulmonary disease (COPD), TANDEM (Tailored intervention for ANxiety and DEpression Management in COPD): protocol for a randomised controlled trial. Trials 2020; 21: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prescott M, Lilley-Kelly A, Cundill B, et al. Home-based Extended Rehabilitation for Older people (HERO): study protocol for an individually randomised controlled multi-centre trial to determine the clinical and cost-effectiveness of a home-based exercise intervention for older people with frailty as extended rehabilitation following acute illness or injury, including embedded process evaluation. Trials 2021; 22: 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pals SL, Murray DM, Alfano CM, et al. Individually randomized group treatment trials: a critical appraisal of frequently used design and analytic approaches. Am J Public Health 2008; 98(8): 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murray DM, Taljaard M, Turner EL, et al. Essential ingredients and innovations in the design and analysis of group-randomized trials. Annu Rev Public Health 2020; 41: 1–19. [DOI] [PubMed] [Google Scholar]
- 36. Baldwin SA, Bauer DJ, Stice E, et al. Evaluating models for partially clustered designs. Psychol Methods 2011; 16(2): 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collins CT, Makrides M, McPhee AJ, et al. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N Engl J Med 2017; 376: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 38. Said HM, Gupta S, Vricella LK, et al. Effect of ambient light on the time needed to complete a fetal biophysical profile: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2017; 217: 59–65. [DOI] [PubMed] [Google Scholar]
- 39. Bhide P, Srikantharajah A, Lanz D, et al. TILT: time-lapse imaging trial – a pragmatic, multi-centre, three-arm randomised controlled trial to assess the clinical effectiveness and safety of time-lapse imaging in in vitro fertilisation treatment. Trials 2020; 21: 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. China L, Skene SS, Bennett K, et al. ATTIRE: Albumin To prevenT Infection in chronic liveR failurE: study protocol for an interventional randomised controlled trial. BMJ Open 2018; 8: e023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kahan BC, Forbes AB, Doré CJ, et al. A re-randomisation design for clinical trials. BMC Med Res Methodol 2015; 15: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kahan BC. Using re-randomisation to increase the recruitment rate in clinical trials. Thesis, Queen Mary University of London, https://qmro.qmul.ac.uk/xmlui/handle/123456789/68609 (2020, accessed 6 September 2021). [Google Scholar]
- 43. Makrides M, Gibson RA, McPhee AJ, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 2009; 301: 175–182. [DOI] [PubMed] [Google Scholar]
- 44. Galyean A, Garite TJ, Maurel K, et al. Removal versus retention of cerclage in preterm premature rupture of membranes: a randomized controlled trial. Am J Obstet Gynecol 2014; 211(4): 399.e1–399.e7. [DOI] [PubMed] [Google Scholar]
- 45. Crowther CA, Middleton PF, Wilkinson D, et al. Magnesium sulphate at 30 to 34 weeks’ gestational age: neuroprotection trial (MAGENTA) – study protocol. BMC Pregnancy Childbirth 2013; 13: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crowther CA, Ashwood P, Andersen CC, et al. Maternal intramuscular dexamethasone versus betamethasone before preterm birth (ASTEROID): a multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc Health 2019; 3(11): 769–780. [DOI] [PubMed] [Google Scholar]
- 47. Schendelaar P, Middelburg KJ, Bos AF, et al. The effect of preimplantation genetic screening on neurological, cognitive and behavioural development in 4-year-old children: follow-up of a RCT. Hum Reprod 2013; 28(6): 1508–1518. [DOI] [PubMed] [Google Scholar]
- 48. Hermans FJR, Schuit E, Opmeer BC, et al. Effectiveness of a cervical pessary for women who did not deliver 48 h after threatened preterm labor (Assessment of perinatal outcome after specific treatment in early labor: Apostel VI trial). BMC Pregnancy Childbirth 2016; 16: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pacagnella RC, Silva TV, Cecatti JG, et al. Pessary plus progesterone to prevent preterm birth in women with short cervixes: a randomized controlled trial. Obstet Gynecol 2022; 139: 41–51. [DOI] [PubMed] [Google Scholar]
- 50. Van der Worp H, Zwerver J, Hamstra M, et al. No difference in effectiveness between focused and radial shockwave therapy for treating patellar tendinopathy: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2014; 22(9): 2026–2032. [DOI] [PubMed] [Google Scholar]
- 51. Bengtsson B, Heijl A, Johannesson G, et al. The Glaucoma Intensive Treatment Study (GITS), a randomized clinical trial: design, methodology and baseline data. Acta Ophthalmol 2018; 96(6): 557–566. [DOI] [PubMed] [Google Scholar]
- 52. Bilezikian JA, Tenzel PL, Johnson RG, et al. A preliminary evaluation of two different meshes in minimally invasive inguinal hernia surgery. Surg Endosc 2021; 35(3): 1342–1347. [DOI] [PubMed] [Google Scholar]
- 53. Chew EY, Clemons TE, Jaffe GJ, et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology 2019; 126(4): 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity: a randomized study. Ophthalmology 1993; 100: 238–244. [DOI] [PubMed] [Google Scholar]
- 55. Kwon J, Staley C, McCullough M, et al. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg 2018; 68(6): 1744–1752. [DOI] [PubMed] [Google Scholar]
- 56. Hasselmann J, Björk J, Svensson-Björk R, et al. Inguinal vascular surgical wound protection by incisional negative pressure wound therapy: a randomized controlled trial – INVIPS trial. Ann Surg 2020; 271(1): 48–53. [DOI] [PubMed] [Google Scholar]
- 57. Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117: 1064.e35–1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grimshaw J, Campbell M, Eccles M, et al. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 2000; 17(Suppl. 1): S11–S16. [DOI] [PubMed] [Google Scholar]
- 59. Bernardo J, Nowacki A, Martin R, et al. Multiples and parents of multiples prefer same arm randomization of siblings in neonatal trials. J Perinatol 2015; 35: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gauderman WJ, Barlow WE. Sample size calculations for ophthalmologic studies. Arch Ophthalmol 1992; 110(5): 690–692. [DOI] [PubMed] [Google Scholar]
- 61. Glassman AR, Melia M. Randomizing 1 eye or 2 eyes: a missed opportunity. JAMA Ophthalmol 2015; 133: 9–10. [DOI] [PubMed] [Google Scholar]
- 62. Morris CR, Kuypers FA, Lavrisha L, et al. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 2013; 98(9): 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hodgson KA, Owen LS, Kamlin CO, et al. A multicentre, randomised trial of stabilisation with nasal high flow during neonatal endotracheal intubation (the SHINE trial): a study protocol. BMJ Open 2020; 10: e039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Anaissie EJ, Vartivarian S, Bodey GP, et al. Randomized comparison between antibiotics alone and antibiotics plus granulocyte-macrophage colony-stimulating factor (Escherichia coli-derived) in cancer patients with fever and neutropenia. Am J Med 1996; 100(1): 17–23. [DOI] [PubMed] [Google Scholar]
- 65. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2016; 4(1): 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riikonen P, Saarinen UM, Mäkipernaa A, et al. Recombinant human granulocyte-macrophage colony-stimulating factor in the treatment of febrile neutropenia: a double blind placebo-controlled study in children. Pediatr Infect Dis J 1994; 13(3): 197–202. [DOI] [PubMed] [Google Scholar]
- 67. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013; 346: e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pandis N, Chung B, Scherer RW, et al. CONSORT 2010 statement: extension checklist for reporting within person randomised trials. BMJ 2017; 357: j2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hopewell S, Clarke M, Moher D, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med2008; 5: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bryant D, Havey TC, Roberts R, et al. How many patients? How many limbs? Analysis of patients or limbs in the orthopaedic literature: a systematic review. J Bone Joint Surg Am 2006; 88(1): 41–45. [DOI] [PubMed] [Google Scholar]
- 72. LeBrun DG, Tran T, Wypij D, et al. Statistical analysis of dependent observations in the orthopaedic sports literature. Orthop J Sports Med 2019; 7(1): 8818410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roberts C. The implications of noncompliance for randomized trials with partial nesting due to group treatment. Stat Med 2021; 40: 349–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sauerland S, Lefering R, Bayer-Sandow T, et al. Fingers, hands or patients? The concept of independent observations. J Hand Surg Br 2003; 28: 102–105. [DOI] [PubMed] [Google Scholar]
- 75. Sullivan TR, Yelland LN, Moreno-Betancur M, et al. Multiple imputation for handling missing outcome data in randomized trials involving a mixture of independent and paired data. Stat Med 2021; 40: 6008–6020. [DOI] [PubMed] [Google Scholar]
- 76. Moerbeek M, Wong WK. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat Med 2008; 27: 2850–2864. [DOI] [PubMed] [Google Scholar]
- 77. Heo M, Litwin AH, Blackstock O, et al. Sample size determinations for group-based randomized clinical trials with different levels of data hierarchy between experimental and control arms. Stat Methods Med Res 2017; 26(1): 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? BJOG 2004; 111(3): 213–219. [DOI] [PubMed] [Google Scholar]
- 79. Yelland LN, Salter AB, Ryan P, et al. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account? Paediatr Perinat Epidemiol 2011; 25(3): 283–297. [DOI] [PubMed] [Google Scholar]
- 80. Sauzet O, Wright KC, Marston L, et al. Modelling the hierarchical structure in datasets with very small clusters: a simulation study to explore the effect of the proportion of clusters when the outcome is continuous. Stat Med 2013; 32: 1429–1438. [DOI] [PubMed] [Google Scholar]
- 81. Sauzet O, Peacock JL. Binomial outcomes in dataset with some clusters of size two: can the dependence of twins be accounted for? A simulation study comparing the reliability of statistical methods based on a dataset of preterm infants. BMC Med Res Methodol 2017; 17: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kahan BC, White IR, Eldridge S, et al. Independence estimators for re-randomisation trials in multi-episode settings: a simulation study. BMC Med Res Methodol 2021; 21: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hibbs AM, Black D, Palermo L, et al. Accounting for multiple births in neonatal and perinatal trials: systematic review and case study. J Pediatr 2010; 156(2): 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park MS, Kim SJ, Chung CY, et al. Statistical consideration for bilateral cases in orthopaedic research. JBJS 2010; 92: 1732–1737. [DOI] [PubMed] [Google Scholar]
- 85. International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials, 2020, https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical-principles_en.pdf
- 86. Wu Z, Frangakis CE, Louis TA, et al. Estimation of treatment effects in matched-pair cluster randomized trials by calibrating covariate imbalance between clusters. Biometrics 2014; 70(4): 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kilpatrick KW, Hudgens MG, Halloran ME. Estimands and inference in cluster-randomized vaccine trials. Pharm Stat 2020; 19(5): 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Frangakis CE, Rubin DB, Zhou X. Clustered encouragement designs with individual noncompliance: Bayesian inference with randomization, and application to advance directive forms. Biostatistics 2002; 3: 147–164. [DOI] [PubMed] [Google Scholar]
- 89. Kahan B. Re-randomisation trials in multi-episode settings: estimands and independence estimators. Stat Methods Med Res 2022; 31: 1342–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Page MJ, Higgins JPT, Clayton G, et al. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLoS ONE 2016; 11(7): e0159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995; 273: 408–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ctj-10.1177_17407745221146987 for Partially clustered designs for clinical trials: Unifying existing designs using consistent terminology by Kylie M Lange, Jessica Kasza, Thomas R Sullivan and Lisa N Yelland in Clinical Trials