Abstract
Introduction
We have previously described preclinical literature which supports umbilical cord blood-derived cell (UCBC) therapy as an efficacious treatment for perinatal brain injury. However, efficacy of UCBCs may be influenced by different patient population and intervention characteristics.
Objectives
To systematically review the effects of UCBCs on brain outcomes in animal models of perinatal brain injury across subgroups to better understand the contribution of model type (preterm versus term), brain injury type, UCB cell type, route of administration, timing of intervention, cell dosage, and number of doses.
Methods
A systematic search of MEDLINE and Embase databases was performed to identify studies using UCBC therapy in animal models of perinatal brain injury. Subgroup differences were measured by chi2 test where possible.
Results
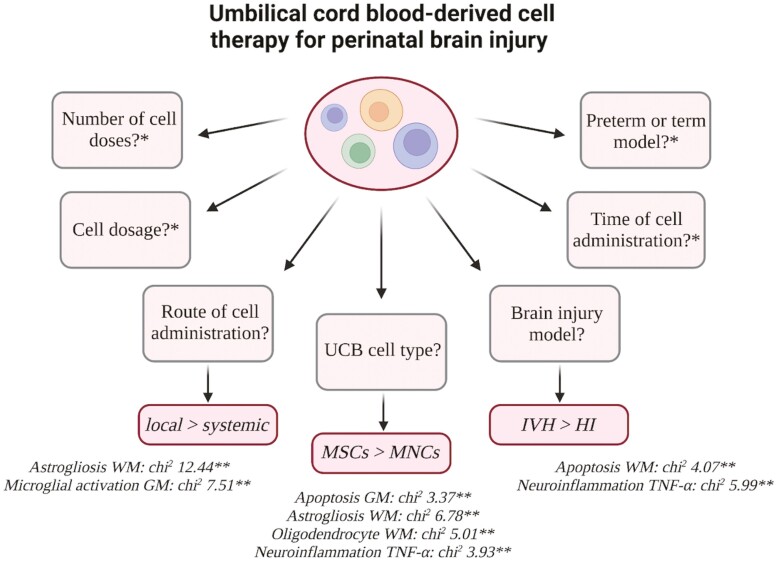
Differential benefits of UCBCs were seen across a number of subgroup analyses including intraventricular hemorrhage (IVH) vs. hypoxia ischemia (HI) model (apoptosis white matter (WM): chi2 = 4.07; P = .04, neuroinflammation-TNF-α: chi2 = 5.99; P = .01), UCB-derived mesenchymal stromal cells (MSCs) vs. UCB-derived mononuclear cells (MNCs) (oligodendrocyte WM: chi2 = 5.01; P = .03, neuroinflammation-TNF-α: chi2 = 3.93; P = .05, apoptosis grey matter (GM), astrogliosis WM), and intraventricular/intrathecal vs. systemic routes of administration (microglial activation GM: chi2 = 7.51; P = .02, astrogliosis WM: chi2 = 12.44; P = .002). We identified a serious risk of bias and overall low certainty of evidence.
Conclusions
Preclinical evidence suggests UCBCs to show greater efficacy in the injury model of IVH compared to HI, the use of UCB-MSCs compared to UCB-MNCs and the use of local administrative routes compared to systemic routes in animal models of perinatal brain injury. Further research is needed to improve certainty of evidence and address knowledge gaps.
Keywords: cord blood stem cell transplantation, fetal blood, brain injuries, perinatal care, systematic review
Graphical Abstract
Graphical Abstract.
Significance Statement.
In neonatal medicine, there is a clear need for the development of new therapies that can provide neuroregenerative benefits for infants with brain injuries. This review offers a unique and comprehensive resource to inform the development of future preclinical and clinical studies. In this review, we systematically reviewed the preclinical literature surrounding UCBCs as a therapy for perinatal brain injury. We investigated the effect variables, such as UCB cell type, timing of administration, and dosage, have on the efficacy of UCB-derived cell therapy in animal models of perinatal brain injury. We identified UCBCs to show greater efficacy in the brain injury model of IVH compared to HI, the use of UCB-derived MSCs compared to MNCs, and the use of local administrative routes compared to systemic routes. In addition to this, we identified knowledge gaps such as the limited preclinical literature surrounding the effect of dose number, sex, and adverse effects.
Introduction
Perinatal brain injury continues to be a major cause of neonatal mortality and life-long neurological disability in both premature and term infants. The term “perinatal brain injury,” understood as brain injury occurring during pregnancy or around the time of birth, encompasses a wide range of neuropathologies.1 These include conditions such as hypoxic ischemic encephalopathy (HIE), intraventricular hemorrhage (IVH), periventricular leukomalacia, and ischemic stroke.1-3 Perinatal brain injury is common across both developed and low to middle-income nations, with certain forms such as HIE having an incidence as high as 2-4 per 1000 live births.4,5 Moreover, perinatal brain injuries are significant contributors to the development of a range of serious neurological sequelae including cerebral palsy (CP), which remains the most common physical disability in childhood.6-8 The high prevalence and morbidity associated with perinatal brain injury highlight the pressing need for developing safe therapies that can effectively reduce and repair brain injuries in infants.
Despite advances in perinatal care which have markedly improved the survival rate of newborns, the available therapies offered for infants born with encephalopathy remains largely supportive.7 The only neuroprotective option available for term born infants with HIE is therapeutic hypothermia.9,10 However, this intervention is only shown to reduce neonatal mortality and major morbidity if started within the first 6 h of life for a period of 72 h and deviation from this protocol has shown to worsen neurological recovery.11 For preterm infants with perinatal brain injury, no current intervention exists except neurosurgical intervention for worsening ventricular dilatation or hydrocephalus following IVH. To see further clinical improvements, new neuroprotective interventions are needed.9-12 Current preclinical interventions under investigation include creatine, melatonin, erythropoietin, xenon, microRNAs, insulin-like growth factors, and stem cell therapies.9,13-15 Umbilical cord blood (UCB)-derived cell therapy is one of the most prominent emerging interventions in this area of research and has received a large amount of attention in both preclinical and clinical studies.16-19
Previously, we demonstrated UCB-derived cells (UCBCs) were effective in improving both neuropathological and behavioral outcomes in preclinical models.20 The specific outcomes investigated were apoptosis, astrogliosis, infarct size, microglial activation, oligodendrocyte number, neuroinflammation, and motor function. Importantly, when we applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool adapted for preclinical studies, the certainty of this evidence was deemed low.21 In this review, we aimed to systematically compare the efficacy of UCB-derived cell therapy on brain outcomes across types of perinatal brain injuries, UCB cell types, routes of intervention, timing of intervention, dosage, and number of cell doses.
Methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and subgroup analysis was performed using a protocol based on The Cochrane Handbook.22,23 The research protocol was registered on PROSPERO (CRD42022275764).
Selection Criteria
Published preclinical studies of any design investigating UCBC therapy for perinatal brain injury were assessed for eligibility. Inclusion criteria consisted of (1) an animal model of perinatal brain injury, (2) an intervention arm that used any UCB cell or subtype, (3) comparator of no intervention or placebo, and (4) assessed structural or functional brain outcomes. Exclusion criteria included non-perinatal brain injury models, adult animal models, non-UCB-derived cells (ie, derived from umbilical cord tissue), and studies that assessed the efficacy of UCBCs in combination with other interventions. Review articles, conference abstracts, studies where full text was not available, and studies unable to be retrieved in English were excluded.
Search Strategy
MEDLINE and Embase databases were searched via Ovid using a combined search strategy conducted by authors, EP and TN. To ensure recent studies were not missed, the search strategy was conducted on June 24, 2021, April 19, 2022, and additional citation searches was performed in August 2022. The advanced search strategy is presented in Supplemental 1.
Study Selection Process
All studies were exported into Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, Australia, available at www.covidence.org). Duplicates were automatically removed using Covidence in conjunction with manual deduplication (EP, TN). Title and abstract screening and full-text screening were independently performed by 2 reviewers (EP, TN). Disagreements were resolved via discussion with a third reviewer.
Data Extraction
Relevant data were independently extracted by 2 review authors (EP, TN). Data extracted included animal species, type of perinatal brain injury, age of injury induction, control details, and intervention characteristics such as cell type, origin species, route of administration, timing of administration, cell dosage, and the number of cell doses administered. When outcome data was published in a figure without tables or text to ascertain values, PlotDigitizer (version 2.6.9) was used to quantify the data. For papers with missing data, specifically standardized mean difference (SMD), n number and standard deviation (SD) or standard error (SE), corresponding authors were contacted a total of 3 times. If authors did not respond, the paper was excluded from the meta-analysis for that particular outcome.
Data Synthesis
Data were synthesized using Review Manager Software for meta-analysis (RevMan, version 5.4). Due to the expected heterogeneity across continuous data measurements, we used a random-effects, inverse variance model to calculate the standardized mean difference (SMD) and 95% CI. The I2 statistic was used to measure heterogeneity, with 25% considered low, 50% considered moderate, and 75% considered high heterogeneity.22
Subgroup Analysis
We aimed to investigate if the intervention effect varied with different patient population and intervention characteristics. Previously, we investigated the brain outcomes of apoptosis, astrogliosis, infarct size, microglial activation, neuron number, oligodendrocyte number, neuroinflammation, and motor function.20 For each brain outcome, we planned to undertake a subgroup analysis of the following pre-specified variables:
Model type
Brain injury type
UCB cell type
Timing of cell administration
Route of cell administration
Cell dosage
Number of cell doses
For each subgroup analysis, we considered the criteria of (i) whether a statistically significant subgroup difference was detected, (ii) the covariate distribution, (iii) the plausibility of the treatment effect, (iv) the importance of the treatment effect, and (v) the possibility of confounding.23 Subgroup differences were measured using the chi2 test, which tested the difference between the pooled effect estimate (ie, SMD) between subgroups. As recommended by The Cochrane Handbook we planned to not compare within-subgroup statistics such as SMDs.22 We defined a statistically significant subgroup effect as one where the covariate considered in the subgroup analysis modified the treatment effect by a P-value less than 0.1 as recommended by The Cochrane Handbook.22 The covariate distribution was taken into account by considering the number of studies and study entries included in each subgroup analysis. The plausibility of the treatment effect was evaluated by considering whether evidence currently existed for the observed treatment effect in different studies of similar interventions. The importance of the treatment effect was considered by acknowledging the size of the measured subgroup difference within the context of the review limitations. Finally, the possibility of confounding was also considered.
As advised by The Cochrane Handbook at least 10 study entries were required for the subgroup meta-analysis to be eligible.22 Additionally, a covariate was defined as a subgroup characteristic that included a minimum of 4 study entries. The covariates included in subgroup analyses are detailed below.
Model Type
Covariates were preterm and term models. Insufficient reporting of preterm and term models was found in studies that used mouse or rat models. Thus, after discussion with review authors, rat preterm was defined as injury induction less than post-natal day (PND) 7 and mouse preterm was defined as injury induction less than PND 9.
Brain Injury Type
All brain injury models were extracted from included studies (chorioamnionitis, excitotoxic brain injury, HI, ischemic stroke, IVH, meningitis, hyperoxia, and FGR). The brain injury model covariates of HI and IVH included a sufficient number of studies for subgroup analysis.
UCB Cell Type
All UCB cell types were extracted from included studies (EPCs, CD34+ cells, CD34− cells, MNCs, monocytes, MSCs, Tregs, and unrestricted somatic stem cells). The UCB cell types of MNCs and MSCs included enough studies to be included as covariates in subgroup analysis.
Timing of Cell Administration
The times of cell administration post-injury induction extracted were grouped as “less than 24 h”, “24-72 h,” and “greater than 72 h.” The covariates of “less than 24 h” and “24-72 h” included a sufficient number of studies for subgroup analysis. Before commencement of the review, we considered how the timing of “early”, “moderate,” and “late” administration times in relation to humans varied across animal species. However, lack of published literature outlining how these differences vary across animals resulted in the team deciding upon the above time ranges across all species. Subsequently, caution should be taken when evaluating the results yielded from the timing of cell administration.
Route of Cell Administration
All routes of cell administration were extracted from included studies (arterial, intracerebral, intranasal, intraperitoneal, intrathecal, intratracheal, intraventricular, and intravenous). The routes of arterial, intraperitoneal, intrathecal, intraventricular, and intravenous underwent subgroup analysis. The following routes of cell administration were combined as covariates to allow for comparison between systemic and local routes of delivery; arterial and intravenous (systemic circulation) as well as intraventricular and intrathecal (local).
Cell Dosage
To allow for the comparison of cell dosage between animal models, cell dose amounts were extracted as cells per kilogram (kg). If studies did not report this unit, reported animal weights for the specific aged animal were used to calculate the cell dose amount. Studies were divided into the 3 covariates of “25 million cells per kg,” “25-100 million cells per kg,” and “greater than 100 million cells per kg.” Before commencement of the review, we considered how cell dosage in relation to humans varied across animal species. For example, we considered how a “low dose” varied in a rat when compared to a sheep. However, the lack of published literature investigating these differences resulted in the aforementioned dose ranges being employed across species. Subsequently, caution should be taken when evaluating the results produced from cell dosage subgroup analyses.
Number of Cell Doses
Covariates included single and multiple cell doses. Insufficient studies used a multiple-cell dose regimen to allow for subgroup analysis to be performed.
Quality Assessment
Two reviewers (EP, TN) independently assessed the risk of bias of included studies using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool.23 Disagreements between reviewers were resolved through discussion with additional authors. Funnel plot analysis in conjunction with Egger’s test was performed to assess the presence of publication bias using MedCalc for Windows, v20.115 (MedCalc Software, Ostend, Belgium). The certainty of evidence was assessed using the GRADE tool adapted for preclinical studies.21
Results
Search Results
A PRISMA flowchart is presented in Supplemental 2. In summary, 1082 citations were identified. After the process of deduplication, 714 papers underwent title and abstract screening using predefined selection criteria. Seventy-two papers underwent full-text screening. Nineteen of these papers were excluded for incorrect population (n = 9), intervention (n = 9), and study design (n = 1). Two additional studies were identified through manual citation searching. After the screening process, a final number of 55 papers were included in this systematic review.
Characteristics of Included Studies
The characteristics of included studies are summarized in Table 1. Studies included preterm (31%) and term (69%) animal models of rats (65%), mice (16%), sheep (13%), and rabbits (6%). The models of brain injury included HI (74%), IVH (13%), ischemic stroke (2%), chorioamnionitis (3%), meningitis (2%), FGR (2%), hyperoxia (2%), and excitotoxic brain lesions (2%). The route of cell administration included systemic circulation (arterial and intravenous) (37%), intraventricular and intrathecal (27%), intraperitoneal (27%), intranasal (3.5%), intracerebral (3.5%), and intratracheal (2%). The timing of brain injury ranged from in utero to PND14. The timing of UCB-derived cell therapy ranged from 1 h to 7 days post-injury induction. UCB cell types included MNC (60%), MSC (17%), CD34+ (10%), EPC (3%), unrestricted somatic stem cells (3%), and others (Tregs, monocytes, CD34− and CD133+). The cell dosage ranged from 0.5 million cells/kg to 800 million cells/kg. Two out of the 55 studies used multiple cell doses of UCBCs.
Table 1.
Characteristics of included studies.
| Study | Model type | Brain injury type | UCB cell type | Cell administration time post-injury | Route of cell administration | Total cells per dose | Number of cell doses |
|---|---|---|---|---|---|---|---|
| Ahn (2013)24 | Preterm | Intraventricular hemorrhage | MSCs | 2 days | Intraventricular | 1 × 105 | 1 dose |
| Ahn (2015)25 | Preterm | Intraventricular hemorrhage | MSCs | 2 days | Intracerebral or intravenous | 1 × 105 (intracerebral) or 5 × 105 (intravenous) | 1 dose |
| Ahn (2018)26 | Term | Meningitis | MSCs | 6 h | Intraventricular | 1 × 105 | 1 dose |
| Ahn (2021)27 | Preterm | Intraventricular hemorrhage | MSCs | 2 days | Intraventricular | 1 × 105 | 1 dose |
| Aridas (2016)28 | Term | Hypoxia ischemia | MNCs | 12 h after birth | Arterial | 1 × 108 | 1 dose |
| Baba (2019)29 | Term | Hypoxia ischemia | MNCs | 21 days | Intravenous | 5 × 106 | 1 dose |
| Bae (2012)30 | Term | Hypoxia ischemia | MNCs | 1 day | Intravenous | 1 × 107 | 1 dose |
| Chang (2021)31 | Term | Hypoxia ischemia | CD34+ or CD34- HSCs | 12 h | Intracerebral | 1 × 105 | 1 dose |
| Cho (2020)32 | Preterm | Hypoxia ischemia | MNCs | 7 days | Intraperitoneal | 3 × 107 | 1 dose |
| Choi (2021)33 | Preterm | Hypoxia ischemia | MNCs | 7 days | Intraperitoneal | 3 × 107 | 1 dose |
| Dalous (2013)34 | Preterm | Excitotoxic brain injury | MNCs | 1 or 24 h (intraperitoneal), 6 or 24 h (intravenous) after birth |
Intraperitoneal or intravenous | 106, 3 × 106 or 107 (intraperitoneal) 106 or 107 (intravenous) |
1 dose |
| De Paula (2009)35 | Term | Hypoxia ischemia | MNCs | 1 day | Intravenous | 1 × 107 | 1 dose |
| De Paula (2012)36 | Term | Hypoxia ischemia | MNCs | 1 day | Intravenous | 1 × 106, 1 × 107 or 1 × 108 | 1 dose |
| Drobyshevsky (2015)37 | Preterm | Hypoxia ischemia | MNCs | 4 h after birth | Intravenous | 2.5 × 106 or 5 × 106 | 1 dose |
| GeiBler (2011)38 | Term | Hypoxia ischemia | MNCs | 1 day | Intraperitoneal | 1 × 107 | |
| Ghaffaripour (2015)39 | Term | Hypoxia ischemia | MNCs | 7 days | Intravenous | 2 × 105 | 1 dose |
| Grandvuillemin (2017)40 | Term | Hypoxia ischemia | MNCs or ECFCs | 2 days | Intraperitoneal | 1 × 107 (MNC) or 5 × 105 (ECFC) | 1 dose |
| Greggio (2014)41 | Term | Hypoxia ischemia | MNCs | 1 day | Arterial | 1 × 106 or 1 × 107 | 1 dose |
| Hattori (2015)42 | Term | Hypoxia ischemia | MNCs | 6 h | Intraperitoneal | 1 × 107 | 1 dose |
| Kadam (2015)43 | Term | Hypoxia ischemia | CD34+ enriched MNCs | 2 days | Intraperitoneal | 1 × 105 | 1 dose |
| Kidani (2016)44 | Preterm | Hypoxia ischemia | CD133+ cells | 1 day | Intraperitoneal | 1 × 105 | 1 dose |
| Kim (2012)45 | Term | Hypoxia ischemia | MSCs | 6 h | Intraventricular | 1 × 105 | 1 dose |
| Kim (2016)46 | Preterm | Hyperoxia | MSCs | PND5 | Intratracheal | 1 × 105 | 1 dose |
| Ko (2018)47 | Preterm | Intraventricular hemorrhage | MSCs | 2 days | Intracerebroventricular | 1 × 105 | 1 dose |
| Li (2014)48 | Preterm | Hypoxia ischemia | MNCs or CD34+ cells | 7 days | Intravenous | 1.5 × 106 | 1 dose |
| Li (2016)49 | Preterm | Hypoxia ischemia | MNCs | 12 h or 5 days | Intravenous | 5 × 107 | 1 dose |
| Li (2017)50 | Preterm | Hypoxia ischemia | MNCs | 12 h | Intravenous | 5 × 107 | 1 dose |
| Li (2018)51 | Preterm | Hypoxia ischemia | MNCs | 12 h | Intravenous | 5 × 107 | 1 dose |
| Lyu (2022)52 | Term | Hypoxia ischemia | MNCs | 1 day | Intravenous | 1 × 107 | 1 dose |
| Malhotra (2020)53 | Preterm | Fetal growth restriction | MNCs | 1 h after birth | Intravenous | 2.5 × 107 | 1 dose |
| McDonald (2018)54 | Term | Hypoxia ischemia | MNCs, Tregs cells, monocytes, EPCs | 1 day | Intraperitoneal | 1 × 106 (MNCs) or 2 × 105 (other) | 1 dose |
| Meier (2006)55 | Term | Hypoxia ischemia | MNCs | 1 day | Intraperitoneal | 1 × 107 | 1 dose |
| Nakanishi (2017)56 | Term | Hypoxia ischemia | Rat MNCs | 3 days | Intraperitoneal | 2 × 106 | 1 dose |
| Ohshima (2016)57 | Preterm | Hypoxia ischemia | CD34+ cells | 2 days | Intravenous | 1 × 105 | 1 dose |
| Park (2015)58 | Term | Hypoxia ischemia | MSCs | 6 h | Intraventricular | 1 × 105 | 1 dose |
| Park (2016)59 | Preterm | Intraventricular hemorrhage | MSCs | 2 or 7 days | Intraventricular | 1 × 105 | 1 dose |
| Paton (2018)60 | Preterm | Chorioamnionitis | MNCs | 6 h | Intravenous | 1 × 108 | 1 dose |
| Paton (2019)61 | Preterm | Chorioamnionitis | MNCs | 6 h | Intravenous | 1 × 108 | 1 dose |
| Penny (2019)62 | Term | Hypoxia ischemia | MNCs | 1 day | Intraperitoneal | 1 × 106 | 1 dose |
| Penny (2020)63 | Term | Hypoxia ischemia | MNCs | 1 day (1 dose group) or 1, 3, 10 days (3 dose group) | Intranasal or intraperitoneal | 1 × 106 | 1 or 3 doses |
| Penny (2021)64 | Term | Hypoxia ischemia | MNCs | 1, 3 and 10 days | Intranasal or intraperitoneal | 1 × 106 | 3 doses |
| Pimentel-Coelho (2010)65 | Term | Hypoxia ischemia | MNCs | 3 h | Intraperitoneal | 2 × 106 | 1 dose |
| Purohit (2021)66 | Preterm | Intraventricular hemorrhage | Unrestricted somatic stem cells | 18 h | Intraventricular | 2 × 106 | 1 dose |
| Rosenkranz (2012)67 | Term | Hypoxia ischemia | MNCs | 1 day | Intraperitoneal | 1 × 107 | 1 dose |
| Rosenkranz (2013)68 | Term | Hypoxia ischemia | MNCs | 1 day | Intraperitoneal | 1 × 107 | 1 dose |
| Tsuji (2014)69 | Term | Ischaemic stroke | CD34+ cells | 2 days | Intravenous | 1 × 105 | 1 dose |
| Vinukonda (2019)70 | Preterm | Intraventricular hemorrhage | Unrestricted somatic stem cells | 18 h | Intravenous or intraventricular | 1 × 106 (intravenous) or 2 × 106 (intraventricular) |
1 dose |
| Wang (2013)71 | Term | Hypoxia ischemia | MNCs | 1 day | Intraventricular | 3 × 106 | 1 dose |
| Wang (2014)72 | Term | Hypoxia ischemia | MNCs | 1 day | Intraventricular | 3 × 106 | 1 dose |
| Wasielewski (2012)73 | Term | Hypoxia ischemia | MNCs | 1 day | Intraperitoneal or intrathecal | 1 × 107 | 1 dose |
| Xia (2010)74 | Term | Hypoxia ischemia | MSCs | 3 days | Intracerebral | 1 × 105 | 1 dose |
| Yasuhara (2010)75 | Term | Hypoxia ischemia | MNCs | 7 days | Intravenous | 1.5 × 106 | 1 dose |
| Yu (2019)76 | Term | Hypoxia ischemia | MNCs or CD34+ cells | 7 days | Intravenous | 1 × 106 (MNCs) or 1.5 × 104 (CD34+) | 1 dose |
| Zhang (2019)77 | Term | Hypoxia ischemia | MNCs | 1 day | Intraventricular | 1 × 107 | 1 dose |
| Zhang (2020)78 | Term | Hypoxia ischemia | MNCs | 1 day | Intraventricular | 3 × 106 | 1 dose |
Abbreviations: ECFC, endothelial colony forming cells; EPCs, endothelial progenitor cells; HSCs, hematopoietic stem cells; MNCs, mononuclear cells; MSCs, mesenchymal stromal cells; PND, post-natal day; Tregs, T regulatory cells.
Effect of Preterm and Term Models on Efficacy of UCB-Derived Cell Therapy
Four of 8 outcomes that underwent subgroup analysis demonstrated a statistically significant difference in the efficacy of UCB-derived cell therapy between preterm and term models as summarized in Supplemental 3. As shown in Supplemental 4A, microglial activation measured in GM, the test for subgroup differences detected a statistically significant subgroup effect in favor of term models (chi2 = 3.11, P = .08). Five studies (7 study entries) evaluated preterm models and 11 studies (16 study entries) evaluated term models. Thus, the covariate distribution was not concerning for this subgroup analysis. As shown in Supplemental 4B, astrogliosis in GM and Supplemental 4C, infarct size, statistically significant subgroup differences in favor of term models were also detected. However, in both subgroup analyses the covariate distribution was unevenly distributed as 7 of 9 study entries were associated with one study in the preterm subgroup. Thus, conclusions should not be drawn from these subgroup analyses. In contrast, as shown in Supplemental 4D, oligodendrocyte number in WM, a statistically significant difference between preterm and term models was detected in favor of preterm models (chi2 = 14.37, P = .0002). Six studies (8 study entries) assessed preterm models and 3 studies (4 study entries) assessed term models. Thus, the covariate distribution was of minimal concern for this analysis. The remaining 4 outcomes did not show statistically significant differences in the efficacy of UCB-derived cell therapy between preterm and term models (Supplemental 4).
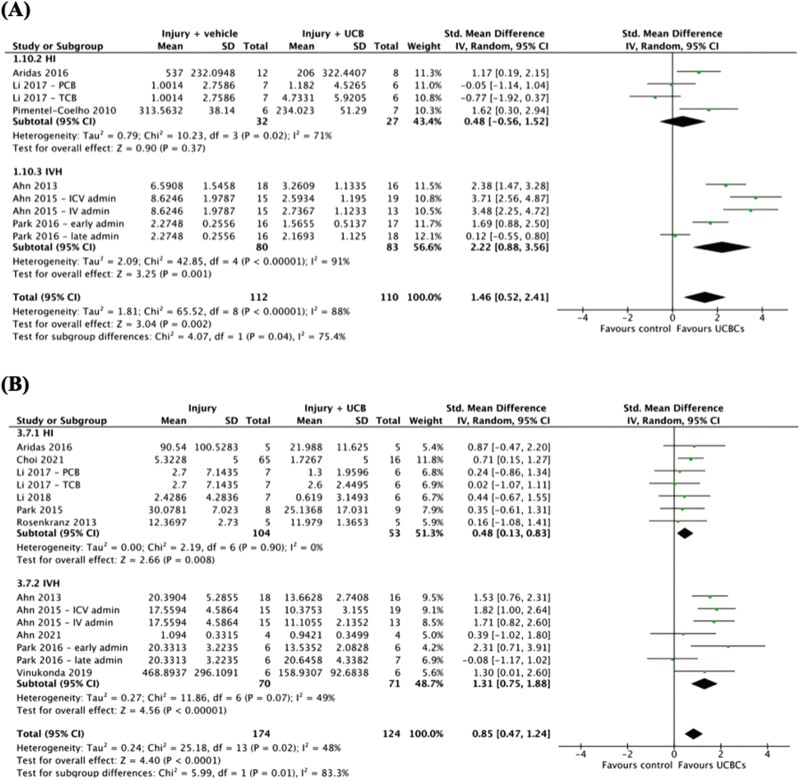
Effect of Type of Brain Injury on Efficacy of UCB-Derived Cell Therapy
As summarized in Supplemental 3, 2 of 2 outcomes that underwent subgroup analysis of brain injury type were associated with a statistically significant subgroup difference in the efficacy of UCB-derived cell therapy. As evident in Fig. 1A, apoptosis in WM, a statistically significant difference between HI and IVH injury models was detected in favor of IVH models (chi2 = 4.07, P = .04). The covariate distribution was not concerning for this analysis as 3 studies (4 study entries) assessed HI and 3 studies (5 study entries) assessed IVH. In a similar fashion, as presented in Fig. 1B, a statistically significant difference in the efficacy of UCB-derived cell therapy on neuroinflammation as measured by TNF-α was found in favor of IVH over HI injury models (chi2 = 5.99, P = .01). Six studies (7 study entries) assessed HI models and 5 studies (7 study entries) assessed IVH models.
Figure 1.
Forest plots demonstrating the effect of brain injury type on brain outcomes of (A) apoptosis—white matter; (B) neuroinflammation—TNF-α. Abbreviations: admin, administration; ICV, intracerebroventricular; IV, intravenous; PCB, preterm cord blood; TCB, term cord blood.
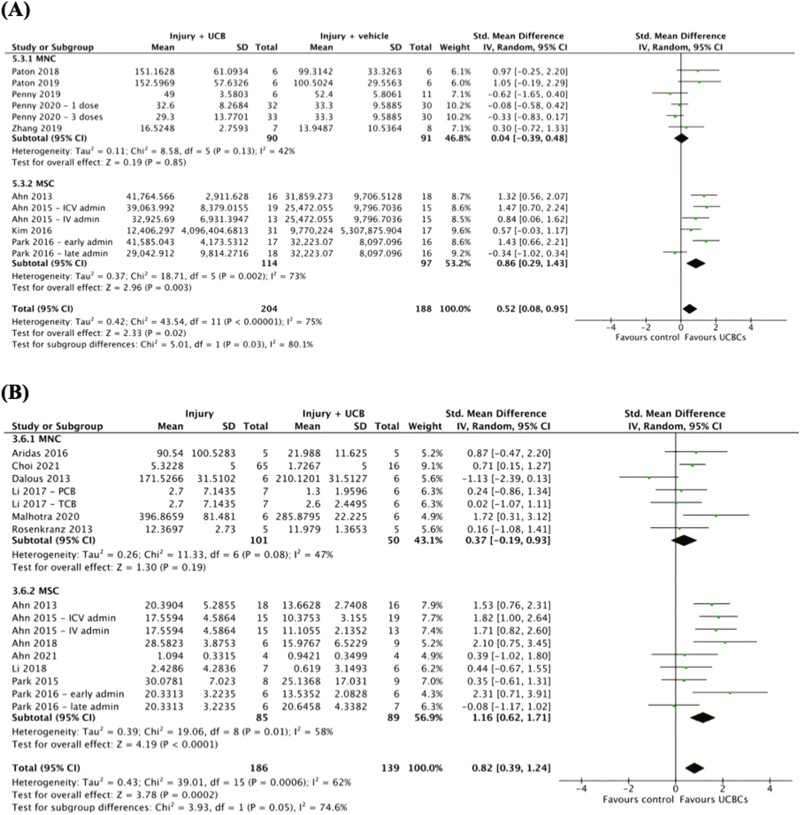
Effect of UCB Cell Type on Efficacy of UCB-Derived Cell Therapy
Four of 8 outcomes demonstrated a statistically significant difference in the efficacy of UCB-derived cell therapy between UCB cell types as detailed in Supplemental 3. As shown in Fig. 2A, a statistically significant subgroup difference between the efficacy of MNCs compared to MSCs when evaluating the outcome of oligodendrocyte number in WM was observed (chi2 = 5.01, P = .03). This modification of the treatment effect was in favor of MSCs. Five studies (6 study entries) evaluated the efficacy of MNCs and 4 studies (4 study entries) evaluated the efficacy of MSCs. Similarly, as evident in Fig. 2B, neuroinflammation as measured by TNF-α, a statistically significant subgroup difference was detected in favor of MSCs over MNCs (chi2 = 3.93, P = .05). The covariate was evenly distributed with 6 studies (7 study entries) investigating MNCs and 7 studies (9 study entries) investigating MSCs. Additional subgroup differences were detected in apoptosis in GM (Supplemental 6A) and astrogliosis in WM (Supplemental 6B). Both of these subgroup analyses also detected statistically significant differences in favor of MSCs over MNCs. The remaining 4 outcomes demonstrated no statistically significant differences in the efficacy of UCB-derived cell therapy between MNCs and MSCs (Supplemental 6).
Figure 2.
Forest plots demonstrating the effect of UCB cell type on brain outcomes of (A) oligodendrocyte number—white matter; (B) neuroinflammation—TNF-α. Abbreviations; admin, administration; ICV, intracerebroventricular; IV, intravenous; MNC, mononuclear cell; MSC, mesenchymal stromal cell.
Effect of Timing of Cell Administration on Efficacy of UCB-Derived Cell Therapy
As summarized in Supplemental 3, 4 of 8 outcomes showed a statistically significant difference in the efficacy of UCB-derived cell therapy across different times of cell administration. As shown in Supplemental 7A, apoptosis WM, a statistically significant modification in treatment effect was seen in favor of cell administration timing of “24-72 h” post-injury induction when compared to “less than 24 h” (chi2 = 4.72, P = .03). The covariate distribution was of moderate concern for this analysis as 6 studies (7 study entries) formed the “less than 24 h” subgroup and 3 studies (4 study entries) formed the “24-72 h” subgroup. As evident in Supplemental 7B, neuroinflammation as measured by IL-1 β, a statistically significant difference in subgroups of “less than 24 h” and “24-72 h” post-injury induction was also seen in favor of’24-72 h’ (chi2 = 3.31, P = .07). The covariate distribution was not concerning for this subgroup analysis. A similar pattern of UCB-derived cell therapy favoring “24-72 h” post-injury induction over “less than 24 h” was also found in astrogliosis in WM and neuroinflammation as measured by TNF-α. These are presented in Supplemental 7. The remaining 4 outcomes analyzed showed no statistically significant differences in the efficacy of UCB-derived cell therapy between different intervention administration times post-injury induction (Supplemental 7).
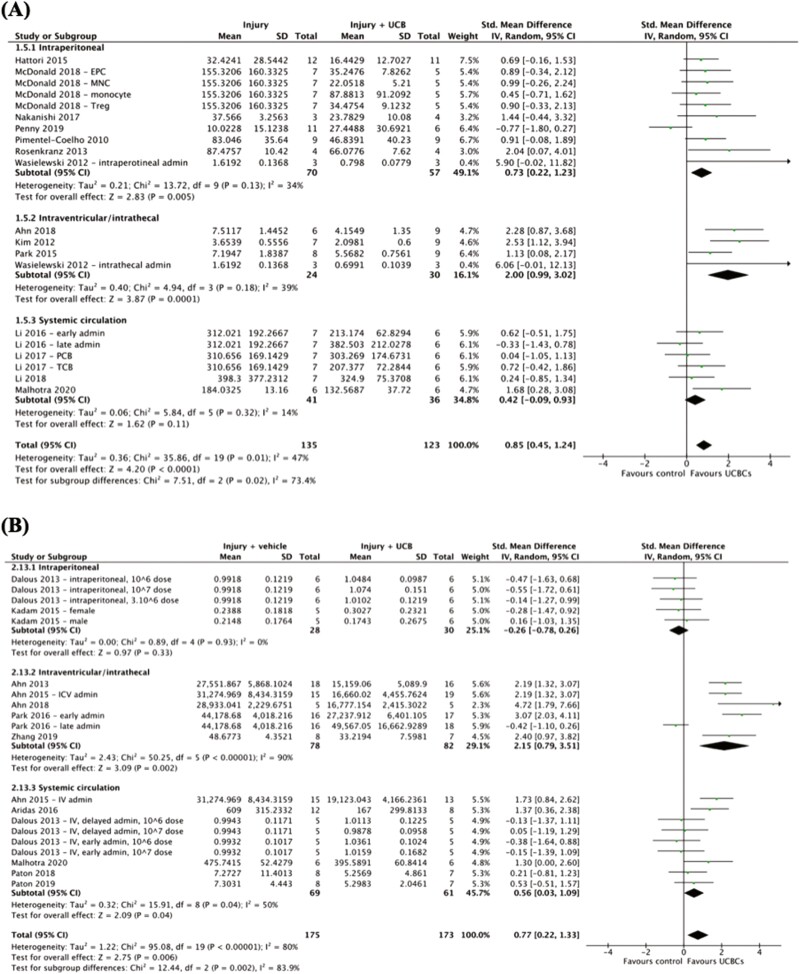
Effect of Cell Administration Route on Efficacy of UCB-Derived Cell Therapy
Three of 8 outcomes were shown to have a statistically significant difference in the efficacy of UCB-derived cell therapy across varying routes of cell administration as summarized in Supplemental 3. As seen in Fig. 3A, microglial activation in GM, the test for subgroup differences detected a statistically significant subgroup effect in favor of intraventricular/intrathecal administration over systemic circulation (chi2 = 7.51, P = .02). A sufficient number of trials were included in the subgroup analysis with 7 studies (10 study entries) contributing to intraperitoneal route of administration, 4 studies (4 study entries) contributing to intraventricular/intrathecal route of administration and 4 studies (6 study entries) contributing to systemic circulation. A similar subgroup effect favoring intraventricular/intrathecal administration over intraperitoneal administration was also detected in astrogliosis in WM (Fig. 3B, chi2 = 12.44, P = .002). In contrast, as shown in Supplemental 8A, motor function measured by cylinder test, a subgroup difference favoring intraperitoneal route of cell administration over intraventricular/intrathecal route was detected (chi2 = 6.50, P = .01). The covariate distribution was not concerning for this subgroup analysis as 6 studies (6 study entries) examined intraperitoneal administration and 4 studies (5 study entries) examined intraventricular/intrathecal route of cell administration. The remaining 5 outcomes demonstrated no statistically significant differences in the efficacy of UCB-derived cell therapy between cell administration routes (Supplemental 8).
Figure 3.
Forest plot demonstrating the effect of route of cell administration on brain outcomes (A) microglial activation—grey matter; (B) astrogliosis—white matter. Abbreviations: admin, administration; EPC, endothelial progenitor cell; ICV, intracerebroventricular; IV, intravenous; MNC, mononuclear cell; PCB, preterm cord blood; TCB, term cord blood; Treg, T regulatory cell.
Effect of Cell Dosage on Efficacy of UCB-Derived Cell Therapy
As summarized in Supplemental 3, 1 of 10 outcomes demonstrated a statistically significant difference in the efficacy of UCB-derived cell therapy with different cell dosages. As shown in Supplemental 9A, apoptosis in WM, a statistically significant subgroup effect was found between “less than 25 million cells per kg” and “25-100 million cells per kg” (chi2 = 5.63, P = .02). This modification in treatment effect favored “less than 25 million cells per kg.” The covariate distribution was not concerning for this analysis as a sufficient number of studies were included in each subgroup. The remaining 9 outcomes which underwent subgroup analysis of dose amount found no statistically significant differences in the efficacy of UCB-derived cell therapy between different doses (Supplemental 9).
Quality Assessment
Quality assessment of the 55 included studies has been previously described.20 The risk of bias of included studies was assessed using the SYRCLE risk of bias tool and is presented in Supplemental 10.79 In summary, most biases assessed were judged “unclear” due to lack of sufficient reporting. Additionally, through further assessment via the generation of funnel plots and Egger’s test, publication bias was assessed as high across brain outcomes. The certainty of results was assessed using the GRADE tool adapted for preclinical studies.21 As previously detailed, after the assessment of risk of bias, inconsistency, imprecision, publication bias, indirectness and upgrading, the overall certainty of evidence for our findings was rated as low.
Discussion
Previously we have concluded UCB-derived cell therapy is an efficacious treatment in preclinical models of perinatal brain injury, with benefits seen across both neuropathological and functional outcomes.20 However, findings were limited by a low certainty of evidence. In this paper, we demonstrated for the first time that variations in study features and design, specifically IVH brain injury, use of UCB-MSCs, and local route (near the site of injury) of administration play a statistically significant role in modifying the treatment effect seen with administration of UCB-derived cell therapy for perinatal brain injury.
Model of Brain Injury
One reason stem cell therapies receive such widespread attention in neonatal research is their unique potential to improve multiple disease states.80 Despite this, our systematic review identified a heavy focus on HI brain injury, with 41 out of 55 studies investigating HI. Due to the limited studies investigating other forms of brain injury, we were only powered to compare the brain injury models of HI and IVH by subgroup analysis. The outcomes which demonstrated a statistically significant difference between models of brain injury were apoptosis in WM and neuroinflammation measured by TNF-α. The data from our meta-analysis suggests that UCBCs may potentially offer more significant neuropathological improvements in IVH injury when compared to the HI model of brain injury. However, it is important to emphasize this insight is heavily limited by the overall low certainty of our evidence. In addition, high heterogeneity across studies also introduces the possibility of confounding. Nonetheless, our paper highlights the need for further studies to investigate the potential of UCBCs as therapy for brain injury models other than HI. Of the 7 studies we identified that assessed the effect of UCBCs on IVH, none were performed in large animal models. Furthermore, there is no current preclinical literature which directly compares the efficacy of UCB-derived cell therapy across different models of brain injury. Thus, further investigation comparing perinatal brain injuries is warranted and may offer insights into the underlying mechanisms of UCBCs as well as provide essential evidence-based data to inform the development of future clinical trials.
UCB Cell Type
UCB refers to blood within the umbilical cord and blood vessels surrounding the fetal component of the placenta.17 Numerous cell types comprise UCB including HSCs, MSCs, Tregs, monocytes, and EPCs.81 In our review, all 4 outcomes which demonstrated statistically significant differences between UCB cell types were associated with a favored modification of treatment effect in MSCs over MNCs. Our review provides further neuropathological support for UCB-MSCs as a potential therapeutic option for infants with perinatal brain injury.13,61,82 To the best of our knowledge, in the current literature no study is yet to directly compare UCB-MNCs to UCB-MSCs. However, Paton et al. (2019) has investigated UCB-MNCs cells to UC-MSCs and found the cell types had differential effects on WM in the preterm brain.61 The results of our review are consistent with the reasoning for this differential effect being that MSCs comprise <0.1% of the total MNCs in UCB.61 The beneficial effects seen in our review are also consistent with a recent systematic review performed by Lehnerer et al. (2022) which found that administration of MSCs (sourced from bone marrow, UCB, placenta, Wharton’s jelly, and adipose tissue) significantly favored sensorimotor and cognitive performance in perinatal arterial ischaemic stroke injured animals.82 Despite our review findings supporting the use of UCB-MSCs over UCB-MNCs, particularly in the context of WM microstructure, it is important to highlight other UCB cell types, such as Tregs and monocytes, did not have sufficient studies to be included in subgroup analysis. Moreover, it is essential to understand our review findings in the context of our quality assessment, which found that the overall certainty of our results was low, primarily due to the high heterogeneity between studies.
Route of Cell Administration
A range of UCB-derived cell therapy delivery routes has been investigated in preclinical literature. UCBCs can be delivered locally around the site of injury (intracerebral, intraventricular, intrathecal, and intranasal) or systemically (intravenous, intraarterial, and intraperitoneal).7 In our review, 3 outcomes showed statistically significant differences between the method of delivery. Two of these outcomes (astrogliosis in WM and microglial activation in GM) favored intraventricular/intrathecal administration over systemic routes and the 3rd outcome (motor function measured by cylinder test) favored intraperitoneal route of cell administration over local routes. These data are suggestive that UCB-derived cell therapy may potentially be more effective on neuropathological outcomes when UCBCs are administered locally to the injured site where they have been shown to have effects via cell-to-cell contact in addition to paracrine mechanisms.9,82,83 In the preclinical space, only 3 studies have directly compared the routes of intracerebral, intraventricular, or intrathecal administration to another route of cell administration. Wasielewski et al. (2012) compared the routes of intrathecal to intraperitoneal, Ahn et al. (2015) compared intracerebral to intravenous, and Vinukonda et al. (2019) compared intraventricular to intravenous routes.25,70,73 Further studies comparing intraventricular or intracerebral routes of delivery to other less invasive local routes such as intranasal delivery and systemic routes would be valuable additions to the current preclinical literature. Additionally, in the clinical setting, the majority of trials have implemented intravenous routes of administration.16 To the best of our knowledge, there has been one phase one trial using intraventricular transplantation and this was shown to be safe and feasible in extremely premature infants with severe IVH.84 In comparing this trial to clinical trials performed in children with cerebral palsy, intrathecal, and intraventricular delivery of stem cells have also been shown to have no inferior safety profile to systemic routes in early phase trials.85,86 Thus, further research is needed to be done to investigating the safety profile, feasibility, and efficacy of local administration routes of UCBCs.
Limitations
We acknowledge there are limitations to this review. Of most importance is the high heterogeneity within the studies investigated. Included studies varied across animal species, brain injury models, UCB cell types, administration routes, cell dosage, measurement tools, and animal sex. Although such heterogeneity enabled subgroup analyses to be performed, the substantial heterogeneity significantly reduced the overall certainty and validity of the evidence. For example, across different animal species an early cell administration time point and high cell dose amount in relation to humans varies considerably. Subsequently, caution should be taken when evaluating the results yielded from timing of cell administration and cell dosage. Additionally, the significant heterogeneity between studies also introduced the possibility for interactions between variables to occur. For instance, when comparing MSCs to MNCs, the dose range, animal species, and injury type varies across studies and thus the possibility of confounding variables is a significant limitation. However, due to the limited number of studies within each subgroup analysis, the ability to explore such interactions was not feasible in this review. Additionally, despite our best efforts to retrieve missing data from respective authors, a number of studies were excluded from respective meta-analyses due to missing data. Thus, as previously discussed, through GRADE analysis the overall certainty of evidence is considered low due to factors such as heterogeneity and serious risk of bias seen across studies.20
Furthermore, our review included distinct treatment groups of the same study as individual study entries. Although this method has been implemented across several past reviews, when a limited number of study entries exist within a subgroup, the effect seen in one particular study can substantially influence the overall SMD seen for that subgroup.22 Similarly, when evaluating the results of this review caution should be taken when subgroups included a limited number of studies. Important to note is 26 of the 44 subgroup analyses performed found no statistically significant differences. To determine if there is indeed a lack of significant differences in these factors or if the review was limited by insufficient power, future meta-analyses incorporating a larger number of studies and homogeneity between studies are needed. In addition to this, the size of subgroup differences detected should also be noted. Fifteen of the 18 subgroup analyses which detected a statistically significant subgroup difference were measured as a P value between .001 and .1. By incorporating a larger number of studies and minimizing heterogeneity across studies, our results and findings may have altered.
Another limitation was that both preterm and term injury models were combined in this review. A differential effect was seen in the subgroup analyses which compared preterm and term models. Microglial activation in grey matter demonstrated a statistically significant difference in favor of term models while oligodendrocyte number measured in white matter demonstrated a statistically significant difference in favor of preterm models. One explanation for this observation is that white matter injury is the most common type of brain injury seen in preterm infants.49 Subsequently, combining both preterm and term models in subgroup analyses such as timing of administration and cell type, is a significant limitation of this review. Furthermore, subgroup analyses of cell dose number, sex, and adverse effects were not evaluated in this review. Recent literature has shown administration of multiple cell doses is an important factor in the efficacy of UCBCs.63 However, due to the limited number of studies which implemented a multiple-dose regimen, we were not powered to undertake a subgroup analysis. Similarly, we found most studies in the review did not comment on the safety profile or potential adverse effects of UCBCs. Investigating the safety profile of UCBCs is essential for further progression in clinical research and thus is recommended to be a focus of future preclinical studies.
Additionally, a major limitation of this review was that functional outcomes were restricted to motor function as measured by rotarod and cylinder tests. Other clinically important functional outcomes such as cognitive function were unable to be investigated due to lack of preclinical literature investigating such outcomes and wide variation in measurement tools used across studies. This is a significant limitation as statistical differences in neuropathological biomarkers identified may not correlate with corresponding differences in functional outcomes. Further preclinical research into functional outcomes in addition to standardization of how such outcomes are measured is recommended. This will enable future meta-analyses of functional outcomes to be performed and thus allow for more robust preclinical evidence to inform future clinical research. Finally, in this review we limited our focus to UCBCs. It is important to note there are other sources of cells that have shown potentially neuroregenerative effects such as cells derived from umbilical cord tissue, bone marrow, amnion, and placental tissue.8,12
Future Directions
With the increasing number of clinical trials showing beneficial results, the use of UCB-derived cell therapy in the treatment of infants with brain injuries is an exciting possibility. However, this review has demonstrated that further preclinical research is warranted to progress UCB-derived cell therapy along the research pipeline. We recommend continued research of UCB-derived cell therapy in the context of preterm versus term models, physical sex, brain injury models other than HI such as IVH, cell types other than MNCs, timing of administration particularly greater than 72 h post-injury, effect of local routes of administration such as intranasal compared to other local and systemic routes, the effect of cell dosage, the use of multiple cell doses. Additionally, research into functional outcomes and potential adverse effects of UCBCs should be further investigated and performed in large animal models where feasible. To improve the quality of preclinical evidence, we recommend future studies to pre-register study protocol, adopt standardized tests for measuring functional outcomes, report methodology in greater detail such as use of blinding, randomisation, animal sex, survival rate, dosage in cell/kg, sample numbers, and specify error bars as SD or SEM. In addition to this, future research should investigate how across species we define a preterm or term model, low to high cell dosages, and early to late timing of interventions. Forming standardized definitions of such characteristics across animal species will greatly improve the power of future systematic reviews and yield further needed evidence. In summary, further preclinical research into UCB-derived cell therapy for perinatal brain injury is needed to determine and confirm optimal cell type, timing of administration, route of administration, cell dosage, and dose number across varying brain injuries.
Conclusions
This systematic review and meta-analysis of 55 preclinical studies identified UCBCs to show greater efficacy in the brain injury model of IVH compared to HI, the use of UCB-derived MSCs compared to MNCs, and the use of local administrative routes compared to systemic routes. Additional preclinical research, particularly in large animal models, is required so that we can further identify and confirm differences in the efficacy of UCB-derived cell therapy across all investigated variables in addition to dose number, sex, and adverse effects. Research in such areas is crucial to aid in the translation of UCB-derived cell therapy to the clinical setting.
Supplementary Material
Contributor Information
Elisha Purcell, Department of Paediatrics, Monash University, Melbourne, Australia.
Timothy Nguyen, Department of Paediatrics, Monash University, Melbourne, Australia.
Madeleine Smith, The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
Tayla Penny, The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
Madison C B Paton, Cerebral Palsy Alliance Research Institute, & Speciality of Child and Adolescent Health, The University of Sydney, Sydney, Australia.
Lindsay Zhou, Department of Paediatrics, Monash University, Melbourne, Australia; The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Monash Newborn, Monash Children’s Hospital, Melbourne, Australia.
Graham Jenkin, The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
Suzanne L Miller, The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
Courtney A McDonald, The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Department of Obstetrics and Gynaecology, Monash University, Melbourne, Australia.
Atul Malhotra, Department of Paediatrics, Monash University, Melbourne, Australia; The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, Australia; Monash Newborn, Monash Children’s Hospital, Melbourne, Australia.
Funding
S.M., C.M., and A.M. receive funding from the National Health Medical Research Council, Australia and Cerebral Palsy Alliance, Australia for their research.
Conflict of Interest
G.J. is a member of Generate Life Sciences Inc. (USA) Scientific Advisory Board and received a remuneration. Generate Life Sciences Inc., and their subsidiary company Cell Care Australia, who provide a research grant, do not have any involvement in the preparation or content of this manuscript. All of the other authors declared no potential conflicts of interest.
Author Contributions
E.P. and T.N.: conception and design, literature searching, collection and/or assembly of data, data analysis and interpretation, risk of bias assessment, manuscript writing. M.S.: conception and design, data analysis and interpretation, risk of bias assessment, manuscript editing. T.P., M.P., L.Z., G.J and S.M.: conception and design, manuscript editing. C.M. and A.M.: conception and design, literature searching, data analysis and interpretation, risk of bias assessment, manuscript editing, supervision.
Data Availability
All datasets and analyses created in this review are available from the authors upon reasonable request.
References
- 1. Novak CM, Ozen M, Burd I.. Perinatal brain injury. Clin Perinatol. 2018;45(2):357-375. https://doi.org/ 10.1016/j.clp.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 2. Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11:192-208. https://doi.org/ 10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNally MA, Soul JS.. Pharmacological prevention and treatment of neonatal brain injury. Clin Perinatol. 2019;46(2):311-325. [DOI] [PubMed] [Google Scholar]
- 4. Hagberg H, Edwards,AD, Groenendaal F.. Perinatal brain damage: the term infant. Neurobiol Dis. 2016;92(Pt A):1022-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Placha K, Luptakova D, Baciak L, Ujhazy E, Juranek I.. Neonatal brain injury as a consequence of insufficient cerebral oxygenation. Neuro Endocrinol Lett. 2016;37(2):79-96. [PubMed] [Google Scholar]
- 6. Larroque B, Ancel P-Y, Marret S, et al. ; EPIPAGE Study group. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371(9615):813-820. https://doi.org/ 10.1016/S0140-6736(08)60380-3 [DOI] [PubMed] [Google Scholar]
- 7. Peng X, Song J, Li B, Zhu C, Wang X.. Umbilical cord blood stem cell therapy in premature brain injury: opportunities and challenges. J Neurosci Res. 2020;98:815-825. https://doi.org/ 10.1002/jnr.24548 [DOI] [PubMed] [Google Scholar]
- 8. McDonald CA, Fahey MC, Jenkin G, Miller SL. Umbilical cord blood cells for treatment of cerebral palsy; timing and treatment options. Paediatr Res. 2018;83:333-344. [DOI] [PubMed] [Google Scholar]
- 9. Davidson JO, Dean JM, Fraser M, et al. Perinatal brain injury: mechanisms and therapeutic approaches. Front Biomed Technol. 2018;23(12):2204-2226. [DOI] [PubMed] [Google Scholar]
- 10. Saw CL, Rakshasbhuvankar A, Rao S, Bulsara M, Patole S.. Current practice of therapeutic hypothermia for mild hypoxic ischaemic encephalopathy. J Child Neurol. 2019;34(7):402-409. https://doi.org/ 10.1177/0883073819828625 [DOI] [PubMed] [Google Scholar]
- 11. Davies A, Wassink G, Bennet L, Gunn AJ, Davidson JO.. Can we further optimize therapeutic hypothermia for hypoxic-ischemic encephalopathy? Neural Regen Res. 2019;14(10):1678-1683. https://doi.org/ 10.4103/1673-5374.257512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wagenaar, N, Nikboer CH, van Bel, F.. Repair of neonatal brain injury: bringing stem cell-based therapy into clinical practice. Dev Med Child Neurol. 2017;59(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 13. Cho KHT, Xu B, Blenkiron C, Fraser M.. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 2019;10:227. https://doi.org/ 10.3389/fphys.2019.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Razak A, Hussain A.. Erythropoietin in perinatal hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinat Med. 2019;47(4):478-489. https://doi.org/ 10.1515/jpm-2018-0360 [DOI] [PubMed] [Google Scholar]
- 15. Alonso-Alconada D, Alvarez A, Arteaga O, Martínez-Ibargüen A, Hilario E.. Neuroprotective effect of melatonin: a novel therapy against perinatal hypoxic-ischaemia. Int J Mol Sci. 2013;14(5):9379-9395. https://doi.org/ 10.3390/ijms14059379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou L, McDonald C, Yawno T, et al. Umbilical cord blood and cord tissue-derived cell therapies for neonatal morbidities: current status and future challenges. Stem Cells Transl Med. 2022;11(2):135-145. https://doi.org/ 10.1093/stcltm/szab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xi Y, Yue G, Gao S, Ju R, Wang Y.. Human umbilical cord blood mononuclear cells transplantation for perinatal brain injury. Stem Cell Res Ther. 2022;13:458. https://doi.org/ 10.1186/s13287-022-03153-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuji M, Sawada M, Watabe S, et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Sci Rep. 2020;10:4603. https://doi.org/ 10.1038/s41598-020-61311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuji, M, Sizonenko SV, Baud, O.. Editorial: preventing developmental brain injury – from animal models to clinical trials. Front Neurol. 2019;10:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen T, et al. , Umbilical cord blood-derived cell therapy for perinatal brain injury: a systematic review & meta-analysis of preclinical studies. bioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hooijmans CR, de Vries RBM, Ritskes-Hoitinga M, et al. ; GRADE Working Group. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS One. 2018;13(1):e0187271. https://doi.org/ 10.1371/journal.pone.0187271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deeks JJ, Higgins JPT, Altman DG.. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022).Cochrane; 2022. www.training.cochrane.org/handbook. [Google Scholar]
- 23. Richardson M, Garner P, Donegan S.. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. 2019;7:192-198. [Google Scholar]
- 24. Ahn SY, Chang YS, Sung DK, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44(2):497-504. https://doi.org/ 10.1161/STROKEAHA.112.679092 [DOI] [PubMed] [Google Scholar]
- 25. Ahn SY, Chang YS, Sung DK, et al. Optimal route for mesenchymal stem cells transplantation after severe intraventricular hemorrhage in newborn rats. PLoS One. 2015;10(7):e0132919-e0132919. https://doi.org/ 10.1371/journal.pone.0132919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahn SY, Chang YS, Kim YE, et al. Mesenchymal stem cells transplantation attenuates brain injury and enhances bacterial clearance in Escherichia coli meningitis in newborn rats. Pediatr Res. 2018;84(5):778-785. https://doi.org/ 10.1038/s41390-018-0142-5 [DOI] [PubMed] [Google Scholar]
- 27. Ahn SY, Jie H, Jung W-B, et al. Stem cell restores thalamocortical plasticity to rescue cognitive deficit in neonatal intraventricular hemorrhage. Exp Neurol. 2021;342:113736-113736. https://doi.org/ 10.1016/j.expneurol.2021.113736 [DOI] [PubMed] [Google Scholar]
- 28. Aridas JDS, McDonald CA, Paton MCB, et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb: umbilical cord blood cells for treatment of perinatal asphyxia. J Physiol. 2016;594(5):1421-1435. https://doi.org/ 10.1113/JP271104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baba N, Wang F, Iizuka M, et al. Induction of regional chemokine expression in response to human umbilical cord blood cell infusion in the neonatal mouse ischemia-reperfusion brain injury model. PLoS One. 2019;14(9):e0221111-e0221111. https://doi.org/ 10.1371/journal.pone.0221111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bae S-H, Kong T-H, Lee H-S, et al. Long-lasting paracrine effects of human cord blood cells on damaged neocortex in an animal model of cerebral palsy. Cell Transplant. 2012;21(11):2497-2515. https://doi.org/ 10.3727/096368912X640457 [DOI] [PubMed] [Google Scholar]
- 31. Chang Y, Lin S, Li Y, et al. Umbilical cord blood CD34+ cells administration improved neurobehavioral status and alleviated brain injury in a mouse model of cerebral palsy. Childs Nerv Syst. 2021;37(7):2197-2205. https://doi.org/ 10.1007/s00381-021-05068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho KH, Choi JI, Kim J-O, et al. Therapeutic mechanism of cord blood mononuclear cells via the IL-8-mediated angiogenic pathway in neonatal hypoxic-ischaemic brain injury. Sci Rep. 2020;10(1):4446-4446. https://doi.org/ 10.1038/s41598-020-61441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi JI, Choi J-W, Shim K-H, et al. Synergistic effect in neurological recovery via anti-apoptotic akt signaling in umbilical cord blood and erythropoietin combination therapy for neonatal hypoxic-ischemic brain injury. Int J Mol Sci . 2021;22(21):11995. https://doi.org/ 10.3390/ijms222111995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dalous J, Pansiot J, Pham H, et al. Use of human umbilical cord blood mononuclear cells to prevent perinatal brain injury: a preclinical study. Stem Cells Dev. 2013;22(1):169-179. https://doi.org/ 10.1089/scd.2012.0183 [DOI] [PubMed] [Google Scholar]
- 35. De Paula S, Vitola AS, Greggio S, et al. Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia-ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. Pediatr Res. 2009;65(6):631-635. https://doi.org/ 10.1203/PDR.0b013e31819ed5c8 [DOI] [PubMed] [Google Scholar]
- 36. De Paula S, Greggio S, Marinowic DR, Machado DC, DaCosta JC.. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience. 2012;210:431-441. https://doi.org/ 10.1016/j.neuroscience.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 37. Drobyshevsky A, Cotten MC, Shi Z, et al. Human umbilical cord blood cells ameliorate motor deficits in rabbits in a cerebral palsy model. Dev Neurosci. 2015;37(4-5):349-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. GeiBler M, Dinse HR, Neuhoff S, et al. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS One. 2011;6(6):e20194-e20194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghaffaripour HA, Jalali M, Nikravesh MR, Seghatoleslam M, Sanchooli J.. Neuronal cell reconstruction with umbilical cord blood cells in the brain hypoxia-ischemia. Iran Biomed J. 2015;19(1):29-34. https://doi.org/ 10.6091/ibj.1376.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grandvuillemin I, Garrigue P, Ramdani A, et al. Long-term recovery after endothelial colony-forming cells or human umbilical cord blood cells administration in a rat model of neonatal hypoxic-ischemic encephalopathy. Stem Cells Transl Med. 2017;6(11):1987-1996. https://doi.org/ 10.1002/sctm.17-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greggio S, de Paula S, Azevedo PM, et al. Intra-arterial transplantation of human umbilical cord blood mononuclear cells in neonatal hypoxic–ischemic rats. Life Sci. 2014;96(1-2):33-39. [DOI] [PubMed] [Google Scholar]
- 42. Hattori T, Sato Y, Kondo T, et al. Administration of umbilical cord blood cells transiently decreased hypoxic-ischemic brain injury in neonatal rats. Dev Neurosci. 2015;37(2):95-104. https://doi.org/ 10.1159/000368396 [DOI] [PubMed] [Google Scholar]
- 43. Kadam SD, Chen HG, Markowitz GJ, et al. Systemic injection of CD34+-enriched human cord blood cells modulates poststroke neural and glial response in a sex-dependent manner in CD1 mice. Stem Cells Dev. 2015;24(1):51-66. https://doi.org/ 10.1089/scd.2014.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kidani Y, Miki Y, Nomimura N, et al. The therapeutic effect of CD133+ cells derived from human umbilical cord blood on neonatal mouse hypoxic-ischemic encephalopathy model. Life Sci. 2016;157:108-115. https://doi.org/ 10.1016/j.lfs.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 45. Kim ES, Ahn SY, Im GH, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. 2012;72(3):277-284. https://doi.org/ 10.1038/pr.2012.71 [DOI] [PubMed] [Google Scholar]
- 46. Kim YE, Park WS, Sung DK, et al. Intratracheal transplantation of mesenchymal stem cells simultaneously attenuates both lung and brain injuries in hyperoxic newborn rats. Pediatr Res. 2016;80(3):415-424. https://doi.org/ 10.1038/pr.2016.88 [DOI] [PubMed] [Google Scholar]
- 47. Ko HR, Ahn SY, Chang YS, et al. Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling. Stem Cell Res Ther. 2018;9(1):326-326. https://doi.org/ 10.1186/s13287-018-1052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li X, Shang Q, Zhang L.. Comparison of the efficacy of cord blood mononuclear cells (MNCs) and CD34+ cells for the treatment of neonatal mice with cerebral palsy. Cell Biochem Biophys. 2014;70(3):1539-1544. https://doi.org/ 10.1007/s12013-014-0090-6 [DOI] [PubMed] [Google Scholar]
- 49. Li J, Yawno T, Sutherland A, et al. Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Exp Neurol. 2016;283:179-187. https://doi.org/ 10.1016/j.expneurol.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 50. Li J, Yawno T, Sutherland A, et al. Term vs. preterm cord blood cells for the prevention of preterm brain injury. Pediatr Res. 2017;82(6):1030-1038. https://doi.org/ 10.1038/pr.2017.170 [DOI] [PubMed] [Google Scholar]
- 51. Li J, Yawno T, Sutherland AE, et al. Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia-ischemia. Exp Neurol. 2018;308:120-131. https://doi.org/ 10.1016/j.expneurol.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 52. Lyu H, Sun DM, Ng CP, et al. Umbilical cord blood mononuclear cell treatment for neonatal rats with hypoxic ischemia. Front Cell Neurosci. 2022;16:823320-823320. https://doi.org/ 10.3389/fncel.2022.823320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malhotra A, Castillo-Melendez M, Allison BJ, et al. Neurovascular effects of umbilical cord blood-derived stem cells in growth-restricted newborn lambs. Stem Cell Res Ther. 2020;11(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDonald CA, Penny TR, Pation MCB, et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J Neuroinflammation. 2018;15(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meier C, Middelanis J, Wasielewski B, et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res. 2006;59(2):244-249. https://doi.org/ 10.1203/01.pdr.0000197309.08852.f5 [DOI] [PubMed] [Google Scholar]
- 56. Nakanishi K, Sato Y, Mizutani Y, et al. Rat umbilical cord blood cells attenuate hypoxic-ischemic brain injury in neonatal rats. Sci Rep. 2017;7(1):44111-44111. https://doi.org/ 10.1038/srep44111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ohshima M, Taguchi A, Sato Y, et al. Evaluations of intravenous administration of CD34+ human umbilical cord blood cells in a mouse model of neonatal hypoxic-ischemic encephalopathy. Dev Neurosci. 2016;38(5):331-341. https://doi.org/ 10.1159/000454830 [DOI] [PubMed] [Google Scholar]
- 58. Park WS, Sung SI, Ahn SY, et al. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10(3):e0120893-e0120893. https://doi.org/ 10.1371/journal.pone.0120893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park WS, Sung SI, Ahn SY, et al. Optimal timing of mesenchymal stem cell therapy for neonatal intraventricular hemorrhage. Cell Transplant. 2016;25(6):1131-1144. https://doi.org/ 10.3727/096368915X689640 [DOI] [PubMed] [Google Scholar]
- 60. Paton MCB, Allison BJ, Li J, et al. , Human umbilical cord blood therapy protects cerebral white matter from systemic LPS exposure in preterm fetal sheep. Dev Neurosci. 2018;40(3):258-270. [DOI] [PubMed] [Google Scholar]
- 61. Paton MCB, Allison BJ, Fahey MC, et al. Umbilical cord blood versus mesenchymal stem cells for inflammation-induced preterm brain injury in fetal sheep. Pediatr Res. 2019;86(2):165-173. https://doi.org/ 10.1038/s41390-019-0366-z [DOI] [PubMed] [Google Scholar]
- 62. Penny TR, Sutherland AE, Mihelakis JG, et al. Human umbilical cord therapy improves long-term behavioral outcomes following neonatal hypoxic ischemic brain injury. Front Physiol. 2019;10:283-283. https://doi.org/ 10.3389/fphys.2019.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Penny T, Pham Y, Sutherland AE, et al. Multiple doses of umbilical cord blood cells improve long-term perinatal brain injury. Stem Cells Transl Med. 2020;9(S1):S3. [DOI] [PubMed] [Google Scholar]
- 64. Penny TR, Pham Y, Sutherland AE, et al. Umbilical cord blood therapy modulates neonatal hypoxic ischemic brain injury in both females and males. Sci Rep. 2021;11(1):15788-15788. https://doi.org/ 10.1038/s41598-021-95035-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pimentel-Coelho PM, Magalhães ES, Lopes LM, et al. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010;19(3):351-358. https://doi.org/ 10.1089/scd.2009.0049 [DOI] [PubMed] [Google Scholar]
- 66. Purohit D, Finkel DA, Malfa A, et al. Human cord blood derived unrestricted somatic stem cells restore aquaporin channel expression, reduce inflammation and inhibit the development of hydrocephalus after experimentally induced perinatal intraventricular hemorrhage. Front Cell Neurosci. 2021;15:633185-633185. https://doi.org/ 10.3389/fncel.2021.633185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rosenkranz K, Kumbruch S, Tenbusch M, et al. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell Tissue Res. 2012;348(3):429-438. https://doi.org/ 10.1007/s00441-012-1401-0 [DOI] [PubMed] [Google Scholar]
- 68. Rosenkranz K, Tenbusch M, May C, Marcus K, Meier C.. Changes in Interleukin-1 alpha serum levels after transplantation of umbilical cord blood cells in a model of perinatal hypoxic-ischemic brain damage. Ann Anat. 2013;195(2):122-127. https://doi.org/ 10.1016/j.aanat.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 69. Tsuji M, Taguchi A, Ohshima M, et al. Effects of intravenous administration of umbilical cord blood CD34+ cells in a mouse model of neonatal stroke. Neuroscience. 2014;263:148-158. https://doi.org/ 10.1016/j.neuroscience.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 70. Vinukonda G, Liao Y, Hu F, et al. Human cord blood-derived unrestricted somatic stem cell infusion improves neurobehavioral outcome in a rabbit model of intraventricular hemorrhage. Stem Cells Transl Med. 2019;8(11):1157-1169. https://doi.org/ 10.1002/sctm.19-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang X-L, Zhao Y-S, Hu M-Y, et al. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. Brain Res. 2013;1518:26-35. https://doi.org/ 10.1016/j.brainres.2013.04.038 [DOI] [PubMed] [Google Scholar]
- 72. Wang X, Zhao Y, Wang X.. Umbilical cord blood cells regulate the differentiation of endogenous neural stem cells in hypoxic ischemic neonatal rats via the hedgehog signaling pathway. Brain Res. 2014;1560:18-26. https://doi.org/ 10.1016/j.brainres.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 73. Wasielewski B, Jensen A, Roth-Härer A, Dermietzel R, Meier C.. Neuroglial activation and Cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Res. 2012;1487:39-53. https://doi.org/ 10.1016/j.brainres.2012.05.066 [DOI] [PubMed] [Google Scholar]
- 74. Xia G, Hong X, Chen X, et al. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med. 2010;38(2):215-221. https://doi.org/ 10.1515/jpm.2010.021 [DOI] [PubMed] [Google Scholar]
- 75. Yasuhara T, Hara K, Maki M, et al. Mannitol facilitates neurotrophic factor up‐regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14(4):914-921. https://doi.org/ 10.1111/j.1582-4934.2008.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu Y, Yan YZ, Luo Z, et al. Effects of human umbilical cord blood CD34+ cell transplantation in neonatal hypoxic-ischemia rat model. Brain Dev. 2019;41(2):173-181. https://doi.org/ 10.1016/j.braindev.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 77. Zhang J, Yang C, Chen J, et al. Umbilical cord mesenchymal stem cells and umbilical cord blood mononuclear cells improve neonatal rat memory after hypoxia-ischemia. Behav Brain Res. 2019;362:56-63. https://doi.org/ 10.1016/j.bbr.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 78. Zhang MBJ, Chao-Chao S, Guang-Zu L, et al. Transplantation of umbilical cord blood mononuclear cells attenuates the expression of IL-1β via the TLR4/NF-κB pathway in hypoxic-ischemic neonatal rats. J Neurorestoratol. 2020;8(2):122-130. [Google Scholar]
- 79. Hooijmans CR, Rovers MM, de Vries RBM, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43-43. https://doi.org/ 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Trounson A, McDonald C.. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11-22. https://doi.org/ 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 81. Hoang DM, Pham PT, Bach TQ, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272. https://doi.org/ 10.1038/s41392-022-01134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lehnerer VJ, RoidlA>, Romanstik O, et al. Mesenchymal stem cell therapy in perinatal arterial ischaemic stroke: systematic review of preclinical studies. Pediatr Res. 2022:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jiao, Y, Xiao-yan L, Liu, J.. A new approach to cerebral palsy treatment: discussion of the effective components of umbilical cord blood and its mechanisms of action. Cell Transplant. 2019;28(5):497-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ahn SY, Chang YS, Sung SI, Park WS.. Mesenchymal stem cell for sever intraventricular hemorrhage in preterm infants: phase 1 dose-escalation clinical trial. Stem Cells Transl Med. 2018;7(12):847-856. https://doi.org/ 10.1002/sctm.17-0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eggenberger S, Boucard C, Schoeberlein A, et al. Stem cell treatment and cerebral palsy: systematic review and meta-analysis. World J Stem Cells. 2019;11(10):891-903. https://doi.org/ 10.4252/wjsc.v11.i10.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sun JM, Kurtzberg J.. The effects of umbilical cord blood and cord tissue cell therapies in animal and human models of cerebral palsy. Cerebral Palsy. 2020:97-110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets and analyses created in this review are available from the authors upon reasonable request.