Scheme 3.
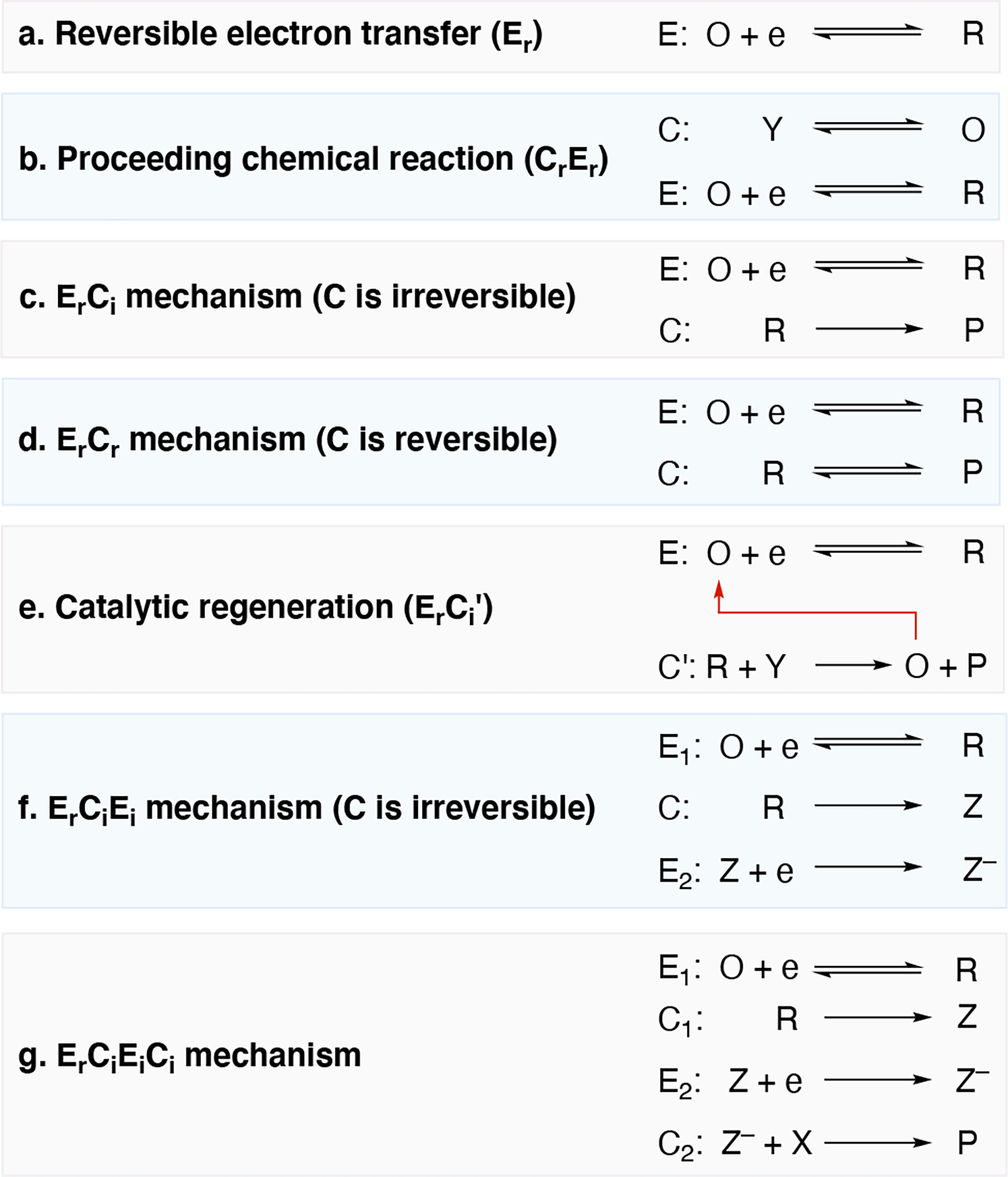
Illustration of various electrode mechanisms. O and R are the oxidized and reduced form of the substrate undergoing a reduction event, e is an electron, Z is a generic intermediate formed during a chemical step, Y and P are a starting material and a product, respectively. The subscripts r and i are used to denote whether the E or C step is reversible or irreversible, respectively. Ci’ denotes an irreversible chemical step that regenerates a catalytic mediator.
