Scheme 10.
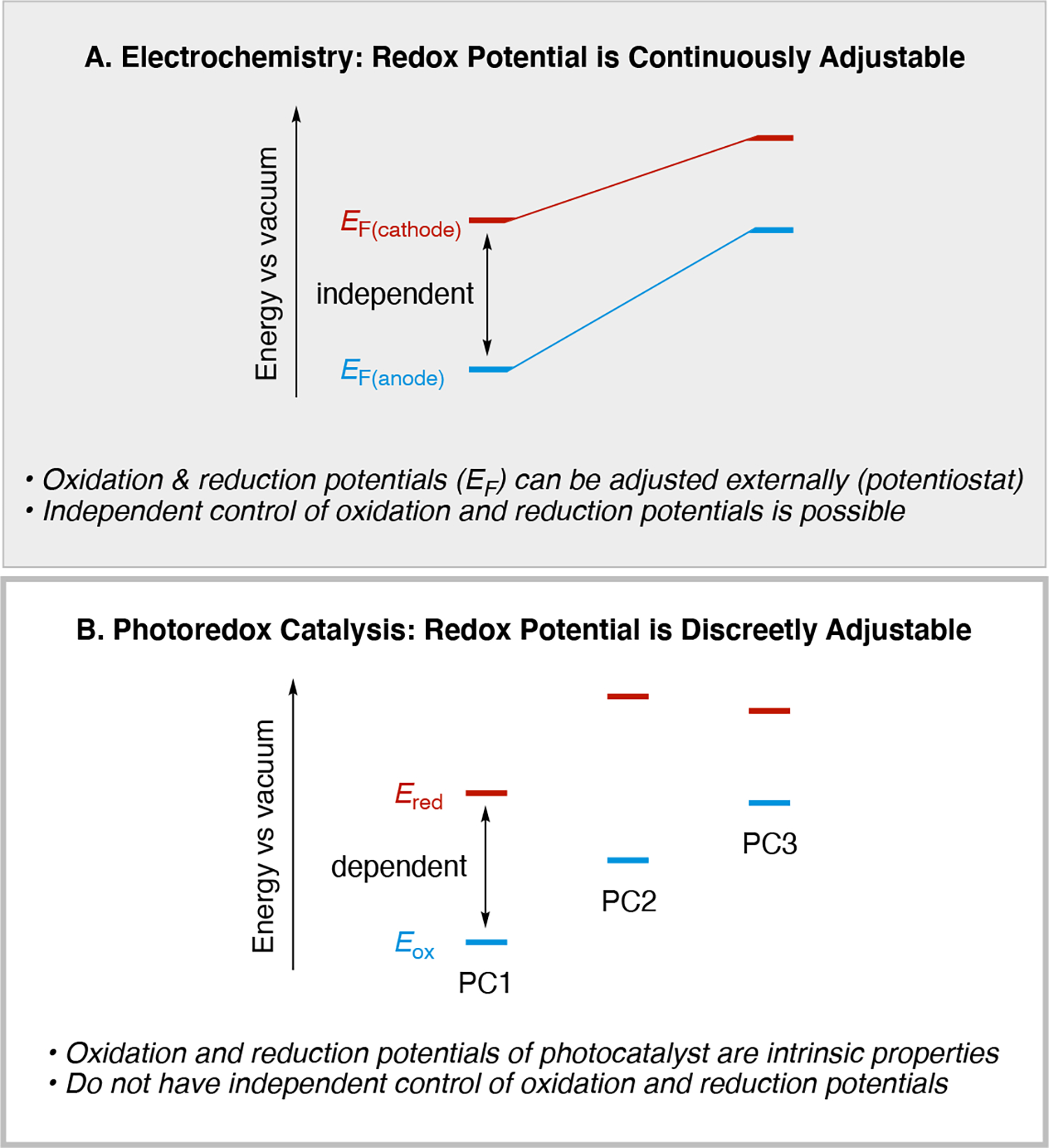
Illustration of how control over the redox potentials differs between (A) electrochemistry and (B) photochemistry. Note: EF(cathode) is the applied potential at the cathode and EF(anode) is the applied potential at the anode. Ered = E(PC*/PC•−) or E(PC•+/PC), and Eox = E(PC*/PC•+) or E(PC•−/PC), depending on the situation.
