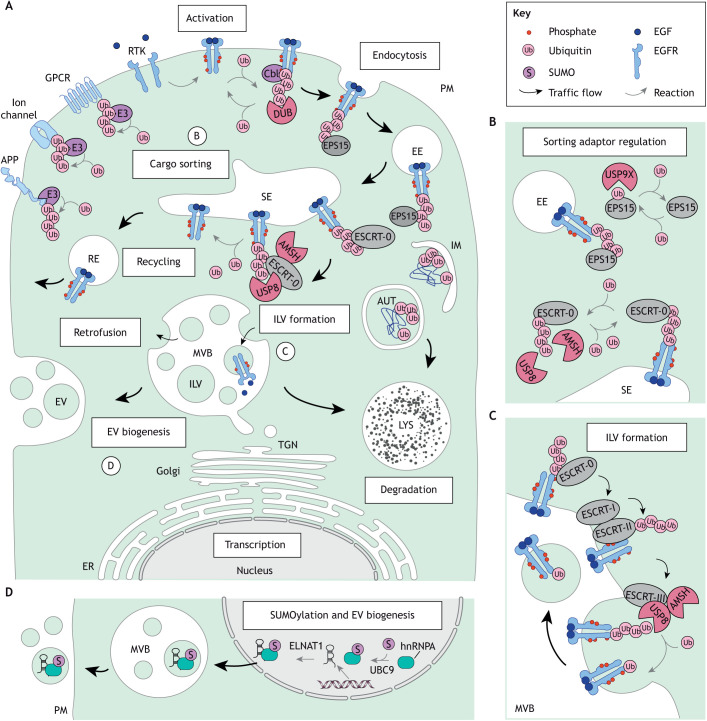
Fig. 1.
Ubiquitylation drives lysosomal turnover of the cell surface proteome. (A) Ubiquitin (Ub) modifications direct cell surface proteins for degradation, as exemplified by the epidermal growth factor receptor (EGFR) whose ligand-induced activation sets in motion its journey to lysosomes. Cargoes endocytosed in early endosomes (EEs) are partitioned between the degradative and recycling membrane pathways at the sorting endosome (SE). Ligand binding triggers EGFR phosphorylation and recruitment of the E3 ubiquitin ligase Cbl for receptor ubiquitylation. Whereas receptors lacking Ub marks traffic back to the plasma membrane (PM) via recycling endosomes (REs), ubiquitylated receptors are recognized by endosomal adaptors and incorporated into intralumenal vesicles (ILVs) of multivesicular bodies (MVBs). Subsequent fusion of MVBs with proteolytic lysosomes (LYS) commits ILV cargoes for degradation. The latter fate can, however, be averted by timely action of DUBs at various steps along the endosomal trafficking route. Besides cargo degradation, other ILV pathways exist, including return to the limiting membrane (LM) via retrofusion and release as extracellular vesicles (EVs). The autophagy pathway also feeds into the endolysosomal system via double-membrane autophagosomes (AUT) that form upon sealing of the isolation membrane (IM) around ubiquitylated cytosolic cargoes. Processes labeled B–D are shown in more detail in the other panels. (B) Ub-dependent functions of endosomal sorting adaptors are regulated by reversible ubiquitylation. Monoubiquitylation of EPS15 inhibits recognition of ubiquitylated cargoes, and deubiquitylation by USP9x restores this function. ESCRT-0 function is also positively regulated by its associated DUBs USP8 and AMSH. (C) Sequestration of ubiquitylated cargoes on ILVs is orchestrated by sequential actions of the ESCRT-0, -I, -II, and -III complexes. ESCRT-associated deubiquitylation during ILV formation ensures Ub homeostasis on endosomal membranes and maintains the cellular Ub pool. (D) SUMOylation of the RNA-binding protein hnRNPA facilitates incorporation of the long noncoding RNA ELNAT1 into ILVs for release in EVs.