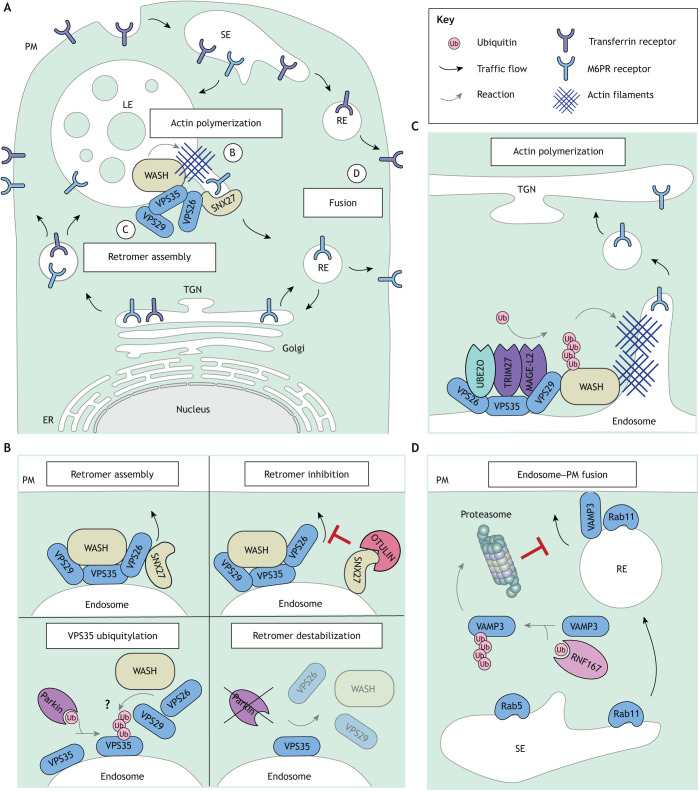
Fig. 2.
Ubiquitylation regulates retromer-mediated recycling. (A) Membrane recycling from maturing endosomes towards the plasma membrane (PM) and the trans-Golgi network (TGN) is carried out by the retromer complex comprising the cargo-selective subunit VPS35 and its partners VPS29 and VPS26. The retromer associates with the WASH complex for actin filament assembly and membrane-deforming sorting nexins (SNX) proteins. Processes labeled B–D are shown in more detail in the other panels. (B) Retromer assembly and stability are regulated by ubiquitylation. The E3 ligase Parkin ubiquitylates VPS35 and stabilizes other retromer components, however, whether ubiquitylation of VPS35 promotes retromer assembly is unclear. Association of the DUB OTULIN with SNX27 antagonizes its binding to VPS26 and thus inhibits retromer-mediated recycling to the PM. (C) Ubiquitylation of the WASH complex, mediated by the retromer-associated E3 ligase MAGE-L2 in conjunction with its substrate adaptor TRIM27 and conjugating E2 enzyme UBE2O, activates localized actin polymerization and promotes recycling from endosomes to the PM. (D) Polyubiquitylation of the SNARE VAMP3 by the E3 ligase RNF167 enhances proteasomal destruction of VAMP3 and inhibits fusion of TGN-derived recycling compartments with the PM.